Editor’s note: This story is featured in the 2022 year in review.
A nature lover from early childhood, Karie Behm found peace and renewal through hiking and cross-country running. She spent much of her time exploring the forested terrain of her native Kansas, as well as Minnesota and Colorado, during family vacations.
She did not suspect that on one unremarkable day, microscopic, corkscrew-like pathogens known as spirochetes would stealthily invade her body, causing a succession of painful, debilitating and perplexing disease symptoms. It would take physicians over six years to untangle the mystery.
Behm had contracted Lyme disease, following a tick bite.
A recent doctoral graduate from the School of Molecular Sciences and the Biodesign Institute at Arizona State University, Behm has devoted her energies to unlocking some of the secrets of Lyme disease, a tenacious ailment affecting some 500,000 Americans every year.
“People have finally come to the realization that Lyme disease has been around a long time and has been affecting a lot of people who have been chronically ill and couldn't get help,” Behm says. “Patient advocacy groups have been working tirelessly for the last 20 years to try to bring attention to this problem. It's not a little thing and it's not a joke. It can affect you your entire life.”
Karie Behm, former researcher with the Biodesign Center for Fundamental and Applied Structural Discovery, now an ORISE Research Fellow at the Center for Disease Control and Prevention in Atlanta.
Behm came to ASU in 2016, with a personal goal in mind — to study the disease causing her often incapacitating symptoms. A highly motivated scholar and researcher, she managed to convince her advisor, Debra Hansen, of the importance of studying this underreported and misunderstood ailment.
Behm and Hansen, both researchers with the Biodesign Center for Applied Structural Discovery (CASD), then consulted Petra Fromme, director of the center and an authority on the structural characterization of proteins, including those associated with infectious disease.
The team hatched a plan to study Lyme arthritis, a pervasive symptom found in many Lyme disease cases. “Petra loves students who share her passion,” Behm says. “During my first interview with her, she got really excited about how the lab’s structural techniques could be applied to Lyme disease.” Prior to her arrival at the lab, Behm, working independently, had already completed an NSF proposal to study Lyme disease.
The project gathered momentum when Behm conducted a literature search, identifying a suite of proteins believed to decorate the surface of Borrelia burgdorferi, the bacterial parasite transmitted by a tick bite that causes Lyme disease.
Drawing on a recent award from ASU Women and Philanthropy, the team is advancing its efforts by using a powerful method known as cryo-EM to observe membrane proteins found on the surface of the Borrelia parasite in stunning detail.
The research has already led to a remarkable discovery. One such membrane protein, known as BBA57, binds to copies of itself to form a complex at the membrane surface. The result appears to be a portal to and from the cell’s interior — a pore. The discovery arrived late one night in the lab, in the form of fresh electron microscope images. It was the culmination of years of dogged research and opens the door to effective therapies against Lyme disease, and potentially other serious afflictions as well.
Of the 100 or so membrane proteins recognized in B. burgdorferi, BBA57 is special. Its presence is linked to Lyme arthritis, a painful, untreatable and often lifelong affliction occurring in 20% of Lyme disease patients. The discovery of the membrane hole may be a turning point, as it provides an ideal site to attack Lyme arthritis by designing a drug that can plug up the pore.
“This project aims to discover the first structure of the major protein responsible for Lyme arthritis, BB57, which forms a pore-like structure in the membrane,” Fromme says. “When we unravel the structural basis for Lyme arthritis, new drugs can be developed to fight the condition and prevent the lifelong suffering of hundreds of thousands of patients."
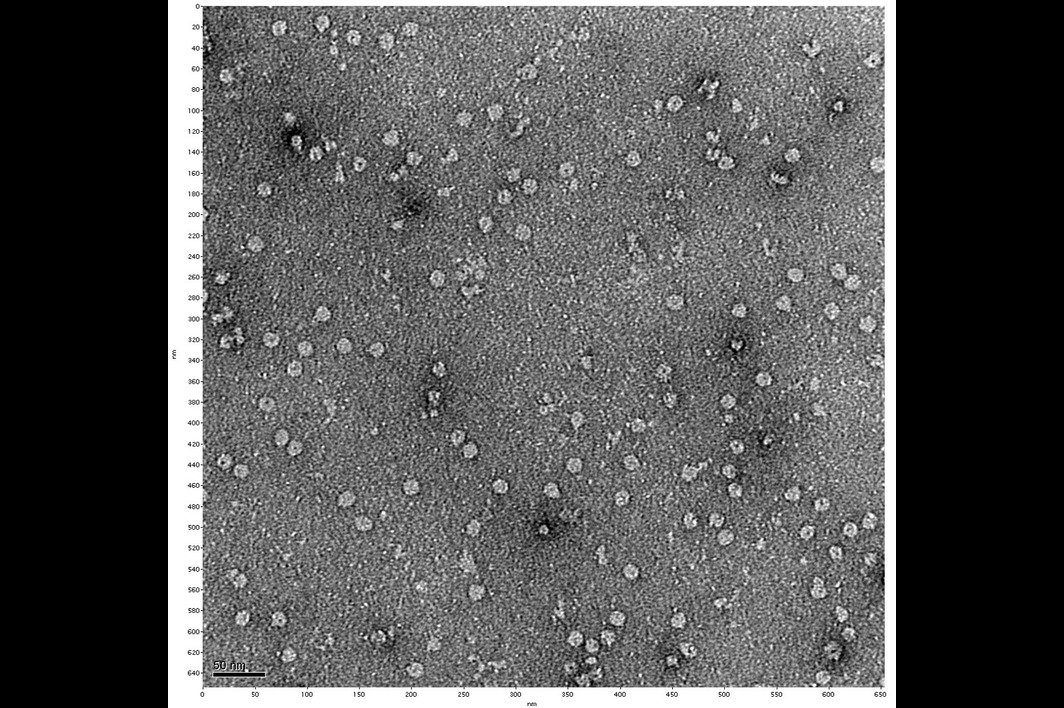
Eureka moment: Behm's negative stain electron microscopy image, offering the first visual evidence of a membrane pore identified by the researchers. This pore may provide an attractive therapeutic target for future drug design against Lyme arthritis. Photo courtesy Biodesign Institute
Climate change and Lyme disease
The bacteria responsible for Lyme disease are carried in the saliva of several tick varieties. In the Northeast and upper Midwestern U.S., the disease is transmitted by the blacklegged tick (Ixodes scapularis), and by the western blacklegged tick (Ixodes pacificus) along the Pacific Coast.
Lyme-carrying ticks operate by stealth. They are not able to fly or jump, instead finding their targets through a process known as questing. Resting atop grasses or shrubs, the ticks hold fast to leaves or grass using their lower legs. The upper pair of legs is kept outstretched, awaiting an unsuspecting passerby. When a suitable host brushes past the place where the tick lies in wait, it quickly climbs onto the host and locates a place to bite and feed on blood.
Ticks may attach themselves to any part of the human body but are often found in well-concealed regions, including the groin area, armpits and scalp. Once a tick has attached and begun feeding, it usually takes 36–48 hours for the Lyme bacteria to be transmitted.
While Borrelia bacteria can cause severe illness in their human hosts, in a diabolical twist, they may improve the tick’s fitness by modifying its central nervous system, extending the length of time the tick spends questing and making it more resistant to extremes of temperature and dryness. To make matters worse, many Lyme disease patients become co-infected with other pathogenic microbes lurking in ticks.
Another area of concern is the fact that reported cases of the disease have roughly doubled since 1991. This is likely due to a combination of factors, including more awareness of the disease and better diagnostic approaches. But there is also mounting evidence that the geographic range over which Lyme-carrying ticks wander may be expanding due to climate change.
Disease of a thousand faces
Many factors may affect the timing and nature of symptoms of Lyme disease, which typically begin three to 30 days following infection. Such symptoms may include fever, chills, headache, fatigue, muscle and joint aches, and swollen lymph nodes, with or without an accompanying rash.
One reason Lyme disease is so vexing to properly diagnose is that it can manifest in many ways in different patients. While 70% of Lyme cases develop the signature circular rash according to reporting, even this figure remains a topic of fierce controversy, and the CDC believes there may be a significant overreporting of rashes. This is because without the rash, Lyme cases are less likely to be diagnosed and treated in a timely manner by physicians.
Should the telltale rash known as erythema migrans occur, it will appear at the site of the tick bite, expanding gradually over several days. The rash may feel warm to the touch but is usually not painful or itchy. The rash sometimes clears as it enlarges, producing a characteristic bullseye appearance, but can assume different shapes, if it is present at all.
An array of further symptoms can develop days or months after infection, including facial palsy; neck stiffness; severe headache; intermittent pain in tendons, muscles, joints and bones; heart palpitations or an irregular heartbeat (known as Lyme carditis); nerve pain and inflammation of the brain and spinal cord; episodes of dizziness; and shortness of breath.
Behm recalls the perplexing constellation of symptoms at the start of her illness: “As a younger teenager, I had random medical issues that came up that just didn't seem to make sense,” she says.
“There were visits to a lot of medical facilities. They knew something was wrong but couldn’t figure out what it was. I got passed around to specialists until I was officially diagnosed my senior year of high school.”
Lyme arthritis can cause severe pain and permanent joint damage
Approximately one in four Lyme disease patients may develop Lyme arthritis, involving chronic joint pain and swelling, particularly the knees and other large joints. The disorder occurs when the Lyme disease bacteria migrate to joint tissues, causing inflammation. If left untreated, Lyme arthritis causes permanent damage to joints. While knees are the most commonly affected area, other large joints may be involved, including the shoulder, ankle, elbow, jaw, wrist or hip.
Following diagnosis, Lyme disease is treated with a course of antibiotics, which can work to eliminate the spirochete bacteria and gradually relieve symptoms. Yet, there is an important caveat: time is of the essence.
“The existing treatments generally don't work if you've been infected more than two to six months prior,” Hansen says.
By this time, “the bacteria have disseminated in your body, and go into a persistent, antibiotic-resistant form. So if you're diagnosed six years later, like Karie, and you're treated for the disease, that treatment probably doesn't work because the bacteria are resistant,” she says.
The result can be a collection of symptoms referred to as Post-Treatment Lyme Disease Syndrome. The causes are varied and remain poorly understood. It may be that the bacterium has changed form and sequestered itself in ways that evade detection. In other cases, autoimmune responses may be involved. To date, no treatment exists for these common complications.
Roughly 70,000 people in the U.S. each year experience lifelong, untreatable Lyme disease. Among these are some 42,000 who develop Lyme arthritis. Currently, physicians have had little to offer such patients, but that may be about to change.
Research mentor Debbie Hansen (left) and Behm. Photo courtesy Biodesign Institute
Protein components of Lyme unmasked
Using electron microscopy, Behm, Hansen and Fromme were able to zero in on a protein that had been identified in previous research as an essential element in the process leading to Lyme arthritis. This work had been carried out by Dr. Uptal Pal, a Lyme disease authority from the University of Maryland, who is now collaborating with the ASU group.
The Lyme disease protein complex at the membrane surface of B. burgdorferi is comparatively large, tricky to crystallize and not easily amenable to X-ray crystallography, the conventional gold standard for protein structural analysis. Instead, a relatively new and powerful method known as cryo-EM, ideal for large proteins, was used.
Cryo-EM, a groundbreaking method for investigating 3D protein shapes, has racked up a long list of impressive achievements since 2017, when its discoverers were awarded the Nobel Prize in Chemistry. Nevertheless, the new study marks the first time it has been applied to the study of Lyme disease.
The technique involves flash-freezing solutions of proteins or other biomolecules in vitreous ice, then bombarding them with electrons to produce images of individual molecules suitable for electron microscopy. The images are used to reconstruct the 3D structure of the molecule, an essential step in understanding how proteins work, how their dysfunction (or use by pathogens) can trigger disease and how drugs may be custom engineered to target them.
Molecular mugshot spurs eureka moment
Peering at the specimens through electron eyes, the researchers began to produce a molecular portrait of the suspect believed to be responsible for Lyme arthritis, through the acquisition of thousands of cryo-EM snapshots.
Research by Pal had already shown that strains of Borrelia engineered to lack BBA57 did not produce Lyme arthritis, though the reasons for this remained obscure. It was only through the work of Behm and her team that a plausible mechanism gradually came into focus.
In addition to complex sample preparation, thousands of images had to be carefully assembled. Often, it seemed the researchers were feeling their way through the dark. But late one evening in the lab, a new set of negative stain electron microscopy images were developed, and the results were riveting. At long last, the team had pictures with just enough resolution to make out what unmistakably appeared as a small hole in the membrane, composed of protein subunits.
It was the break they had been waiting for, after three years of work.
The team had theorized that perhaps Borrelia causes Lyme arthritis by transporting some disease factor from within the cell, out into the extracellular environment. And here, before their eyes, was the transport tunnel likely used as a passageway from inside the cell to outside, into the host’s bloodstream.
“Debbie, Petra and I were all there, having a late-night meeting when we saw the images. And everyone shouted, 'Yay!'” Behm recalls.
The elusive pores they had long suspected had materialized with unmistakable clarity.
The race for a cure
The most gratifying news for the group was not simply that their hard work was rewarded with an important insight into the structure of BBA57, but that the finding suggests a promising approach for preventing Lyme arthritis.
The basic idea is simple: Design a drug that plugs up the hole on the Borrelia membrane surface. But to accomplish this, the protein structure of BBA57 will require much more refinement.
The team has calculated that the images of a million pores will be needed to visualize the protein with sufficient resolution to permit drug design. The researchers are using the new award from ASU Woman and Philanthropy to achieve this feat, collecting the mass of images that will permit structural determination down to the locations of atoms.
Achieving atomic resolution will allow the researchers to see where each of the roughly 800,000 atoms making up each pore-forming molecule are situated.
“We'll know the position of each of those atoms, and that kind of intimate detail is enough to be able to computationally look for drug binders, with existing drug libraries,” Hansen says.
Teaming up with Pal, the researchers are pursuing long-term funding through a $3 million grant proposal to NIH, due to begin as early as 2023. The funding will be contingent on the early results the group is producing now, so the pace of study is hectic.
Once the final structural determination of a given protein has been made, drug companies can take the 3D data and use high-performance computers to begin designing drugs to act on it.
The long and winding road ahead
BBA57 is just one protein active in Lyme disease. Many others exist, and once they have been characterized, may also be targeted by smart drugs. The possibility of preventing Lyme disease altogether may be on the horizon, and the general approach pursued by Behm and her colleagues may be applicable to other tick-borne diseases or, possibly, other spirochete afflictions, such as syphilis, yaws and relapsing fever.
Having suffered through the ups and downs of this enigmatic illness, Behm is doubly committed to finding answers. She describes the early days of her pitched battle with the disease as a mixture of fear and determination.
Behm continues her explorations of Lyme disease at her new position as ORISE Fellow at the CDC in Atlanta.
More Science and technology

Stuck at the airport and we love it #not
Airports don’t bring out the best in people.Ten years ago, Ashwin Rajadesingan was traveling and had that thought. Today, he is an assistant professor at the University of Texas at Austin, but back…

ASU in position to accelerate collaboration between space, semiconductor industries
More than 200 academic, business and government leaders in the space industry converged in Tempe March 19–20 for the third annual Arizona Space Summit, a statewide effort designed to elevate…

A spectacular celestial event: Nova explosion in Northern Crown constellation expected within 18 months
Within the next year to 18 months, stargazers around the world will witness a dazzling celestial event as a “new” star appears in the constellation Corona Borealis, also known as the Northern Crown.…