SARS-CoV-2: A theme and variations

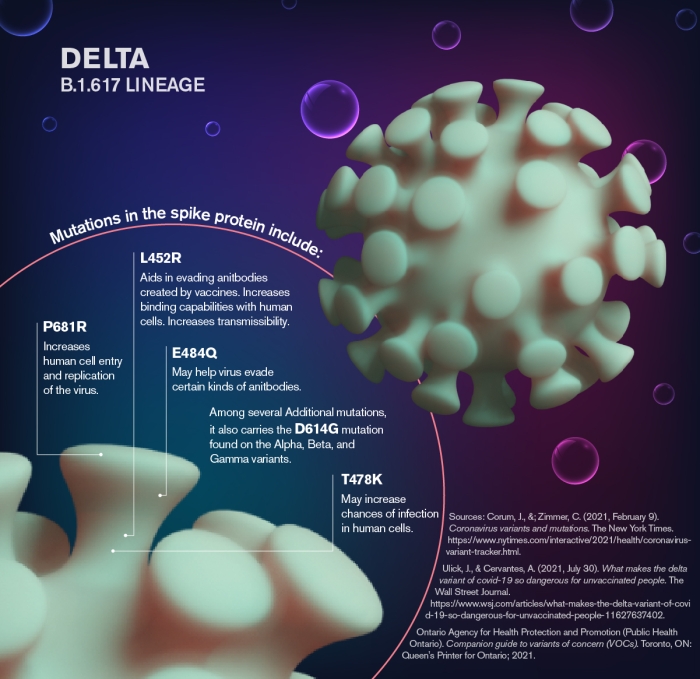
The CDC has identified four "variants of concern," a term applied to variants whose mutations significantly alter the virus’s ability to infect cells, improve viral transmissibility or cause more serious illness. In addition to delta, these variants include alpha, beta and gamma. Graphic by Shireen Dooling
The celebrations were underway. America seemed to be emerging from the depths of the COVID-19 crisis as a vast reduction in infections, hospitalizations and fatalities, driven by improved therapies and a suite of highly effective vaccines, had brought a collective sigh of relief.
Yet just when it seemed the novel coronavirus was finally relaxing its grip on society, the elusive pathogen delivered another surprise. Delta, a highly infectious variant, is now sweeping the nation and the world.
Mirroring trend lines in much of the country, Arizona’s infections and hospitalizations have risen to levels not seen since the winter surge, with the week leading up to Aug. 10 averaging more than 2,000 cases per day. Describing alarming new revelations delivered during an internal Centers for Disease Control and Prevention meeting on July 29, health officials declared “the war has changed.”
How we got here
The past two decades have seen the emergence of three novel betacoronaviruses associated with outbreaks in humans. These include severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002, Middle East respiratory syndrome (MERS-CoV) in 2012 and SARS-CoV-2 in 2019. All three coronaviruses are widely believed to have originated in bats and may have jumped to humans by way of an intermediate vertebrate vector.
Strenuous efforts are underway to keep close tabs on the spread of the SARS-CoV-2, including a massive testing and sequencing regime conducted by researchers with the Biodesign Institute at Arizona State University. Thanks to the combined effects of testing, treatment and vaccines, something resembling normal life seemed to be returning, at least in much of the United States.
SARS-CoV-2, however, has fooled us many times since its first stateside detection in Snohomish County, Washington, on Jan. 20, 2020, and so much remains unknown. Looking back at our recent past offers sobering evidence of just how insidious this still largely mysterious virus is.
Just over a year ago, the national rates of COVID-19 were markedly lower than they are today, causing much of the country to let down its guard in terms of masks and social distancing. Shortly thereafter, the country experienced a devastating third wave; one that would claim hundreds of thousands of additional lives and in many cases, overwhelm health care facilities, deplete reserves of essential personal protective equipment, and tax doctors and nurses to the breaking point. The disease has now claimed over 618,000 lives in the U.S. alone.
One fact is clear. SARS-CoV-2 has a wildcard in its deck: the ability to produce large numbers of genomic variants, some of which could impede accurate diagnoses, reduce vaccine effectiveness and in theory, eventually outwit human immunity altogether, whether naturally acquired or vaccine-induced.
Currently, one highly aggressive variant known as B.1.617.2, or delta, has taken center stage, both globally and in the U.S., where it currently accounts for more than 80% of new cases nationwide and over 90% in some of the hardest-hit areas. After its initial appearance in India, delta has now spread to at least 182 countries.
As Joshua LaBaer, executive director of the Biodesign Institute put it in a recent press conference: “Society has largely decided that it is done with the virus. The virus begs to differ.”
Delta blues
The delta variant has rapidly assumed dominance in many parts of the world, causing new shutdowns across Europe, devastating parts of Asia and threatening dire outcomes on the African continent, where fewer than 2% of the population has been vaccinated.
A series of mutations has made the delta variant two to three times as transmissible as the original form of the virus. Two key factors help account for this heightened transmissibility. As a new study in China demonstrates, delta’s prolific replication in the body’s respiratory tracts produces 10 times the viral load of the highly contagious alpha variant and 1,260 times the viral load produced by the original SARS-CoV-2 virus.
Delta also spreads more quickly, reaching detectable levels and becoming transmissible in an infected individual in only four days, compared to six days for the original coronavirus. Many contagious carriers of the virus remain asymptomatic during transmission. One patient infected with delta will likely spread the infection to an average of six people, compared to the original SARS-CoV-2, which would typically spread to 2 or 3 people.
In addition to delta’s supercharged ability to fuse with cell receptor binding domains, invade and infect human cells, the variant has other ways to improve its success rate, including an ability to switch off the body’s early warning defenses. Delta, as well as the highly transmissible alpha variant, accomplish this in several ways.
In one tactic, the virus uses a protein called Nsp1 to block a cell’s messenger RNA (mRNA) from escaping the cell nucleus and producing proteins used to warn the immune system of impending infection. With its exit channels blocked, the cell can’t release interferons, signaling molecules that are critical in initiating an immune response.
It is not yet known whether the delta variant produces more serious illness than other variants, though studies in Canada (not yet published) and Scotland suggest that people infected with delta are more likely to be hospitalized, while research in Singapore indicates that they are more likely to require oxygen. Although more research is needed to verify these findings, the delta variant has the capacity to infect more people, likely producing more hospitalizations and fatalities, compared with earlier variants.
A sudden change in the weather
The CDC’s recent announcement, concerning breakthrough infections among the vaccinated, brought both good and bad news for the ongoing pitched battle against COVID-19. On the positive side, vaccines continue to show high levels of effectiveness in preventing infection and drastically reducing hospitalizations and deaths. On the other hand, breakthrough infections in vaccinated individuals may not be as rare as originally thought and the cycle threshold — a measure of viral concentrations in the nose and throat — are equivalent for vaccinated and unvaccinated individuals who become infected.
The implication of these findings is that if a vaccinated person does become infected, they can pass the disease to others as readily as an infected person who has not been vaccinated. This crucial finding led the CDC to abruptly change course, recommending face masks be worn in public places, regardless of vaccination status, and that the practice of social distancing be restored.
Perhaps most alarmingly, delta can be spread through even fleeting contact with an infected person. A study in Australia describes a case of transmission of delta occurring in just five to 10 seconds of exposure. Because the delta variant is more contagious, the threshold to reach herd immunity within the population is now higher as well.
In short, SARS-CoV-2 in its delta form is now recognized as one of the most infectious viruses in medical history, more transmissible than SARS, MERS, Ebola, smallpox, seasonal flu or the common cold and possibly, as aggressively contagious as chicken pox.
A world divided
The arrival of vaccines and new therapeutics have been game-changing events. Although the risks of infection and serious illness have fallen drastically for the fully vaccinated, emergent, fast-spreading variants of the virus pose a major threat to unvaccinated populations in the U.S.
Globally, SARS-CoV-2 is poised to deliver a devastating blow to undervaccinated nations with highly vulnerable populations. (Uganda, for example, recorded more deaths in June than total fatalities reported since the start of the pandemic.)
Closer to home, LaBaer notes that the current surge in Arizona can be traced to three key factors: increased social interaction and relaxing of social distancing and mask guidelines; warmer weather that has driven much activity indoors, where the virus can more easily spread; and the arrival of delta, a dramatically more transmissible form of the pathogen. COVID-19, the disease caused by the SARS-CoV-2 virus, is now the leading cause of death in the state.
Nationwide, the daily average of confirmed coronavirus cases has dramatically accelerated in the past month, from about 11,000 per day in late June to a daily average of 118,067 during the week ending Aug. 10. In sparsely vaccinated states, variant-linked cases also have fueled a significant increase in COVID-19-related hospitalizations.
On Aug. 1, a day after Florida recorded the highest number of new cases since the start of the pandemic, the state broke the previous record for current hospitalizations, set over a year ago before the advent of vaccines. Louisiana is likewise experiencing record-breaking hospitalizations for COVID-19 set back in January.
Delta’s skyrocketing ascendance to viral dominance has upended earlier predictions of the trajectory of the pandemic. Perhaps even more worrisome is the fact that the longer the virus circulates in the population, the greater the opportunities for new and potentially dangerous variants, as the virus aggressively explores sequence space in a relentless effort to override immunity and replicate.
A population in the crosshairs
Unlike the previous surges, the latest escalation in serious cases is occurring overwhelmingly among the unvaccinated. If the global availability of vaccines and the pace of vaccination don’t improve dramatically, we may lose more lives to COVID-19 in the era after effective vaccines have brought relief to much of the developed world.
In Africa, deaths have increased 80% during the past month — a shocking figure, given the fact that the overwhelming majority of such deaths are now preventable. Globally, more than 4 million cases, largely driven by the delta surge, were reported to the World Health Organization in the last week of July. To give a sense of the exponential potential of the virus, the WHO says that if the current trend continues, total cases are expected to surpass 200 million in the next two weeks.
The blistering ascendance of delta after an initial appearance in just one country is a case study in the threats posed by viral variants that acquire new talents of infectivity, immune evasion and transmission.
Predictions of delta’s next moves and the possible scale of destruction vary widely and depend on many factors, some, unknowable at this time. What is known, however, is that close to 100 million Americans remain without immunity to SARS-CoV-2, because they have not been previously infected nor vaccinated against the virus. Many of these have fallen victim to a campaign of vaccine disinformation, perpetuated via social media channels and some media outlets. This vulnerable population provides ample fuel for the pandemic to reignite and intensify, with potentially dire consequences.
Such outcomes are not limited to COVID-19-related fatalities but also include an array of after-effects from the virus, ranging from cardiac issues to serious neurological complications. As to the full range, severity and duration of these long-haul COVID-19 symptoms, researchers are still largely in the dark.
Error as inspiration
Since the dawn of life, evolution has been propelled by a kind of carelessness. Successful replication of species relies on the faithful copying of the genetic blueprint or genome. This process, known as transcription, is imperfect, with a variety of copying mistakes possible. (In the case of coronaviruses like SARS-CoV-2, the genome is composed of RNA rather than DNA.)
In some cases, the errors arise when proofreading mechanisms tasked with monitoring transcription of the DNA or RNA code, fail. Such mistakes can take several forms. One of the four nucleotides may be accidentally mistranscribed, for example. A letter in the code may be dropped altogether or extraneous letters added, like an unreliable voice-to-text program.
Both foreign adversaries like viruses and human cells that turn rogue in the process of cancer development use mutations in their genomes as secret weapons to outwit the defensive fortifications of their hosts.
While most mutations are useless or harmful to the mutating organism, a tiny subset may, by sheer chance, provide a survival advantage. These mutations can then become fixed in successive generations and gain strength against therapeutics designed to thwart them, including vaccines.
The mutations resulting from inaccurate transcription of the DNA or RNA code occur in all forms of life, to varying degrees. They are responsible for a vast range of diseases and some of them, including SARS-CoV-2, are potentially lethal. But mutations are also the wellspring for the astonishing diversity observed in nature, both within and between species.
Since the start of the pandemic, the world’s population has been peppered with variants of the SARS-CoV-2 virus, though only a handful have reached the eyes and ears of the public, due to concerns about their potential to rapidly spread or reduce the effects of COVID-19 vaccines.
According to the CDC, four variants are labeled “variants of concern,” a term applied to variants whose mutations significantly alter the virus’ ability to infect cells, improve viral transmissibility or cause more serious illness. In addition to delta, these variants include alpha, beta and gamma. (See accompanying graphics).
An evolving threat
Michael Lynch, an evolutionary biologist and director of the Biodesign Center for Mechanisms in Evolution, cites the rapid rise of multiple SARS-CoV-2 variants as predictable given the enormous global population now affected by the virus.
“Every nucleotide site in the genome must be mutating to every alternative nucleotide type every day, many, many thousands of times per day, so the main issue is the arrival time of such variants to high frequency,” Lynch said.
Pathogens use mutations to try to gain an adaptive edge over the body’s immune defenses. According to Lynch, however, the high rate of mutation in RNA viruses like SARS-CoV-2, may provide an Achilles heel, a way to beat the virus at its own game.
“RNA viruses have high mutation rates, up to a million times higher than their hosts, and these high rates are correlated with enhanced virulence and evolvability, traits considered beneficial for viruses,” Lynch said. “However, their mutation rates are almost disastrously high, and a small increase in mutation rate can cause RNA viruses to go locally extinct.”
This may be particularly true for coronaviruses like SARS-CoV-2, which have comparatively large genomes and therefore, may be more vulnerable to infidelities in transcription.
Lynch has suggested this vulnerability could potentially be exploited. The idea would be to deliver a drug capable of interfering with or disabling viral proofreading mechanisms, leading to a catastrophic shower of harmful mutations, which eventually drive the viral population in a given host extinct, through a process known as “mutational meltdown.”
Mass surveillance
Efrem Lim, assistant professsor in the Biodesign Center for Fundamental and Applied Microbiomics studies viral evolution, exploring the ways these entities interact with their human hosts during early development and under various conditions of immunity. To accomplish this, the Lim lab combines molecular virology and bioinformatic approaches.
“My lab studies new viruses, so when people are sick with unknown etiology, we take clinical samples to sequence and identify new pathogens. We also study the microbiome, a very diverse collection of microbes in our body,” Lim said. “It was an easy transition for us to take the technology we have and pivot towards helping us understand this pandemic and the trajectory that SARS-CoV-2 is evolving towards.”
Lim grew up in Singapore, when a closely related pathogen responsible for the respiratory illness SARS began its path of destruction in 2003. The knowledge gained in studying this earlier coronavirus would prove invaluable for researchers combatting SARS-CoV-2, particularly the discovery that SARS-CoV-2 attaches itself to cells by means of the same receptor used by the original SARS virus, known as ACE2.
Lim has demonstrated that SARS-CoV-2 bears another similarity to the agent of the original SARS outbreak in Asia — a large genomic deletion, modifying a crucial protein known as ORF7a, reminiscent of the deletion mutation that occurred in the SARS virus.
The finding, which drew national attention, was reported in the Journal of Virology and co-authored with Biodesign Institute colleagues LaRinda A. Holland, Emily A. Kaelin, Rabia Maqsood, Lily I. Wu and Arvind Varsani, from the Center for Fundamental and Applied Microbiomics; Brenda G. Hogue and Bereket Estifanos from the Center for Immunotherapy, Vaccines and Virotherapy; and Rolf U. Halden and Matthew Scotch, from the Center for Environmental Health Engineering.
The hard-won insights gained from the study of the SARS virus would play a central role in the eventual development of highly effective vaccines for SARS-CoV-2.
“We knew the parts of the virus spike protein and which specific amino acids bind to the ACE2 receptor. We could easily map that on to the new virus SARS-CoV-2 and could focus our attention on developing a vaccine against that region,” Lim said. “Now imagine a world where we did not know that ACE2 was the receptor for CoV-2. How would you start developing your vaccine?”
Rapid response team
When the SARS-CoV-2 pandemic struck in the U.S., Lim and his colleagues launched aggressive efforts to better understand and prevent SARS-CoV-2 transmission. Combining next-generation sequencing studies of SARS-CoV-2 to identify transmission patterns and functional studies to characterize viral mutations in clinical isolates, the researchers began amassing data.
Among their achievements, Lim and his colleagues are responsible for sequencing the first SARS-CoV-2 cases in Arizona. They also identified a recent byproduct in the ongoing biological arms race, a viral variant that first appeared within the state, now known as B.1.243.1.
The Arizona variant, which has since spread to Texas and New Mexico, carries the E484K mutation, sometimes called the “eek” mutation, which has also cropped up in other notable variants, including those first detected in South Africa and Brazil, as well as a variant discovered in New York.
When a virus develops the same mutation independently in different populations and rises in prevalence, it is a good indication that the mutation is not a random hitchhiker in the genome but is somehow beneficial to the virus. In the case of E484K, studies have shown that it affects the virus’s spike protein, altering its electrostatic interactions and therefore its binding affinity with host cell receptors.
Additionally, the E484K mutation appears to blunt the immune response to the virus.
“The E484K mutation has been shown to reduce antibody responses from monoclonal antibodies that are used as therapies and also reduces the neutralization of post-vaccination serum,” Lim said.
Surveillance efforts of the novel pathogen across Arizona are being carried out by state health authorities, in close collaboration with Arizona State University’s Biodesign Clinical Testing Laboratory, which uses advanced, high-throughput technologies to test saliva samples across Arizona.
An extensive and growing repository of samples testing positive for SARS-CoV-2 gave Lim and his colleagues the raw material for the next step — sequencing of viral genomes to identify variants and explore their prevalence and distribution.
“With the (Biodesign Clinical Testing Laboratory) testing at the statewide level through multiple sites all across Arizona, we have a pretty good representation of cases all across our state,” Lim said.
Sequence power
In the race against the variants, speed is crucial. This dual ability to noninvasively test saliva and rapidly sequence positive samples for variant analysis has made the Biodesign Institute a national leader in the battle against SARS-CoV-2. Samples are sequenced at accelerated pace, because the positive tests only need to be sent up one flight at the institute to the sequencing lab, with rapid turnaround times for data processing.
“It's very rare for a single institution to have both the testing capability and next-gen sequencing under one roof,” Lim said.
By the time other institutions have shipped out positive COVID-19 tests for further analysis, the sequencing has already been completed in-house at the Biodesign Institute.
Lim stresses the fact that focusing on a given variant of concern — one which shows increased transmissibility, virulence or treatment resistance — may miss the big picture. Mutations are often cumulative in their effects, and this is the essence of the ominous threat that they pose. One variant may increase a virus’s resistance to vaccines by 5% but combined with another mutation, this can rise to 10%, then 20% and so on. Should enough variants accrue, there is a risk of the virus fully escaping the vaccine. The longer SARS-CoV-2 circulates among unvaccinated people, the greater the risk of new and potentially dangerous variants emerging.
In addition to national efforts, global tracking of viral dissemination based on sequence evolution over time will play a critical role in determining how the virus is changing and where it is going. ASU Biodesign Institute researcher Matthew Scotch is an authority in this rarified discipline, known as phylogeography, and has investigated the complex geographic movements of influenza and other viruses.
Using sequence changes over time and space, phylogeographers gain valuable insights into species histories and explore crucial questions in evolution, including gene flow, rates of mutation, fragmentation, range expansion and colonization. The approach has been used to monitor many human diseases, including dengue fever, rabies, influenza and HIV.
Nature’s defenses meet nature’s intruder
SARS-CoV-2 is fragmenting into a range of variants, the effects of which are under intense study. To further complicate the picture, the virus can behave very differently, depending on the subtle bulwark of human defenses exquisitely designed by evolution to thwart such pathogens — the immune system.
In a recent study, Karen Anderson, Abhishek Singharoy and their colleagues in the Center for Personalized Diagnostics, explore MHC-1, a critical protein component of the human adaptive immune system.
The protein is polymorphic, taking a variety of forms, which differ in their ability to bind SARS-CoV-2 peptides. An analysis of 23 countries showed a close correlation between MHC-1 alleles that more successfully attached to components of the virus and favorable health outcomes.
Such research has important implications for assessing vulnerability to COVID-19 and its emerging variants in both individuals and populations. It may also point the way to future vaccines better able to target essential snippets of the pathogen that more effectively stimulate a robust immune response.
“Immune responses to the vaccines include both B cell (antibody) and T cell immunity,” Anderson said. “Right now, according to the CDC, the current vaccines work on the circulating variants, but more study is needed to understand the duration of effective immunity and the resilience of the current vaccines to emerging variants.”
Globe-trotting menace
SARS-CoV-2 has now reached every continent, migrating with its human hosts as far as Antarctica, where Biodesign virologist Arvind Varsani is exploring the vulnerability of the continent’s varied fauna since the pathogen’s arrival on the frigid, windswept expanses and its possible impact on Antarctica’s fragile ecosystem. The research into SARS-CoV-2’s evolution in nonhuman species is yet another critical piece of the pandemic puzzle.
Reciprocal transmission of SARS-CoV-2 between humans and animals can potentially create wild reservoirs of the virus, endangering long-term control of COVID-19 in people as well as subjecting vulnerable animal populations to a lethal disease.
New variants of the SARS-CoV-2 virus evolving in animal hosts could potentially escape the immunity conferred by current human vaccines. Over the long-term, animal-based reservoirs of SARS-CoV-2 increase the likelihood of disease resurgence, further undermining global disease control.
Coda
After significant progress in controlling the global SARS-CoV-2 pandemic, the nation and the world find themselves returning to a place both disturbing and all-too-familiar — a place of uncertainty. Public health officials express concern about the spread of delta in coming weeks and look to the fall, winter and beyond with trepidation.
Yet, since the start of the pandemic, society has gained an enormous amount of knowledge about the virus and most remarkably, developed the means to defeat it. This will require not only heightened vigilance and ramped-up testing and sequencing, but a thorough reexamination of vaccine equity and the rapid distribution of vaccines to low-income countries.
As Tedros Adhanom Ghebreyesus, director-general of the World Health Organization recently said: “The pandemic will end when the world chooses to end it. It is in our hands.”
More Science and technology

US Department of Energy selects ASU and DCX to pioneer new ways to power data centers
The U.S. Department of Energy has selected Arizona State University and DCX USA, LLC, as key research partners for its…

From the lab to your headphones, new podcast brings ASU research to you
What do you get when an evolutionary biologist, an engineer and an anthropologist walk into a recording studio?At Arizona State…

Lessons on maintaining your humanity in the world of AI technology
AI is not human. But it does a good job of acting like it.It is capable of replicating how we speak, how we write and even how we…