Recently, dramatic news came out of Africa concerning Ebola, one of the world’s deadliest and most feared diseases. New drugs can overcome the virus and save lives.
Health officials from the World Health Organization (WHO) and National Institutes of Health (NIH) announced that four antidotes — including one first developed by Arizona State University and its commercial partners — had been tested in the largest Ebola clinical trial to date. The trial proved so successful, it was ended early to make the best performing antidotes widely available for the ongoing Ebola crisis in the Democratic Republic of the Congo (DRC).
This is the first time that dramatic evidence has been obtained to show improved survival rates in people who had already been infected by the deadly virus. The trial also showed that the earlier health care workers can administer the antidotes after an Ebola infection, the more potential lives can be saved. This included survival rates as high as 96% for one particular antidote.
Planting the roots of discovery
The WHO trial in the DRC began last November. One of the drugs that served as the “control” was ZMapp, which had its discovery origin in work by ASU scientist Charles Arntzen in a collaboration with San Diego-based Mapp Biopharmaceutical and Kentucky Bioprocessing.
The roots of the amazing Ebola cure can be traced back to Charles Arntzen’s groundbreaking research center at ASU’s Biodesign Institute, where he also served as the founding director. Arntzen, who retired from ASU in 2017, had worked closely for several years with Mapp Biopharmaceutical on the idea of plant-based therapeutics to fight infectious diseases, which are still the world’s No. 1 killer.
After 9/11 and the 2001 anthrax attack in the U.S. Senate office building, the government invested heavily in biodefense, including $3.7 million in 2002 to Arntzen and a small San Diego-based startup called Mapp Biopharmaceutical, led by Larry Zeitlin and Kevin Whaley.
“We had proposed to the U.S. Army that plants (specifically tobacco) could be used for rapid production of vaccines or monoclonal antibodies that might be necessary to protect us from bioterrorism. This was shortly after terrorist attacks on New York and the Pentagon in 2001, and the use of anthrax spores for a bioterror attack in the U.S. Senate building; funding for countermeasures was quickly becoming available.”
The goal was to develop defenses against pathogens, including Ebola, that could be used to thwart potential bioterrorist attacks using infectious agents.
“I think the real gain is from all of the money that was invested early on. It takes a long time to build up that core competency that is necessary for drug development,” Arntzen said. “This has happened for both vaccines and therapeutics in academia and companies. We should give credit to funding agencies like DARPA and NIH for giving us the tools that we need.”
Such an endeavor was true to the spirit and mission of the newly formed ASU Biodesign Institute.
George Poste, who succeeded Arntzen as director of the Biodesign Institute and has extensive experience in combating global infectious disease, commented, “Arntzen’s research emphasizes the importance of strong national defense spending on global health and preparedness against constantly emerging new infections such as Ebola, Zika, SARS and MERS."
Using nature as inspiration, Biodesign Institute scientists have taken on major world problems and solved them using highly creative, multidisciplinary teams and formed spinouts or partnered with companies to rapidly move these ideas to the marketplace.
With a dream team of collaborators, Arntzen’s team created a “molecular toolbox” to transform tobacco plants into biological factories. Within three years after proposing this very novel concept to U.S. Army collaborators, the team achieved production of anti-Ebola monoclonal antibodies. Three antibodies with high levels of virus inactivation were including in the Ebola antidote called ZMapp.
“We’ve been teaming together manufacturing innovation, tobacco engineering innovation, our virus work and antibody discovery,” Arntzen said. “Just in the development of ZMapp, I’m guessing there were as many as 100 different people with many different skills who came together.”
After a decade of hard work, sweat and trial-and-error experiments, they made steady progress. Back-to-back ASU/Mapp publications in 2011 described their first success in protecting animals from Ebola infection using plant-made vaccines or antibodies. In work published in 2014, the ZMapp therapeutic cocktail proved to be 100% effective in protecting animals against Ebola, even five days after onset of infection.
After their success in the animal studies, they imagined it would take at least another 5-7 years to do the necessary human clinical trials and gain FDA approval for any widespread human use.
Poste said that “this success in developing effective treatments for Ebola illustrates the complexity of the process of translating innovation from the laboratory to clinical benefit which requires integration of diverse scientific and clinical skills and investment of hundreds of millions of dollars”.
A day that changed the world
Then Aug. 4, 2014, turned into a day that astounded Arntzen.
During the height of the Ebola outbreak, two American missionaries became infected. Physician Kent Brantly and health care worker Nancy Writebol, both near death and desperate for help, became the first people to receive ZMapp, knowing full well that it had never been tested — for safety or effectiveness — in humans before.
“I opened an email from Larry Zeitlin on this morning in August. It was a short message that alerted me to the fact that ZMapp had been used to treat two missionaries in Liberia, and they were recovering from severe Ebola infection.”
“By that afternoon, the newswires were alive with stories with titles such as ‘Secret serum likely saved Ebola patients.’ It was hardly a secret to Larry and me, or our colleagues who had been working for over a decade on finding a life-saving Ebola therapeutic.”
It seems ironic that ZMapp is a serum extracted from tobacco — a plant with a notorious reputation as a killer. The pathway from discovery to treatment began with an idea Arntzen had to produce low-cost vaccines in plants to fight devastating infectious diseases in the developing world. It was one of those outside-the-box ideas that turned into a career-crowning achievement for Arntzen, and an excellent example of success in an academic, federal and industry collaboration.
“What can happen in biology, rarely but wonderfully when it does, is the application of some aspect of research in a way that saves lives,” Arntzen said when he reflected on the events upon his retirement.
It was like a climactic scene out of a sci-fi movie. Within 24 hours after taking ZMapp, Brantly went from death’s door to walking again, and both Writebol and Brantly fully recovered.
“On Aug. 4, in 2014, I was amazed and delighted,” Arntzen said. “We were able to draw a straight line from a hypothesis to a dramatic outcome, which had happened in just over a decade. Such an occurance is rare for a biological scientist like me. I'm still amazed but delighted.”
No one was more moved than Kent Brantly. With a second chance at life, and after five years of a “spiritual and emotional recovery,” he’s now returning to Africa as a medical missionary.
“Since my recovery, I've had the chance to learn the miraculous history of this drug's development,” Brantly said. “I'm grateful for the role the Biodesign Institute at ASU has played in the discovery process and in forging ties to industry collaborators who translated new ideas into the product that I received.”
Thwarting an ever-alarming epidemic
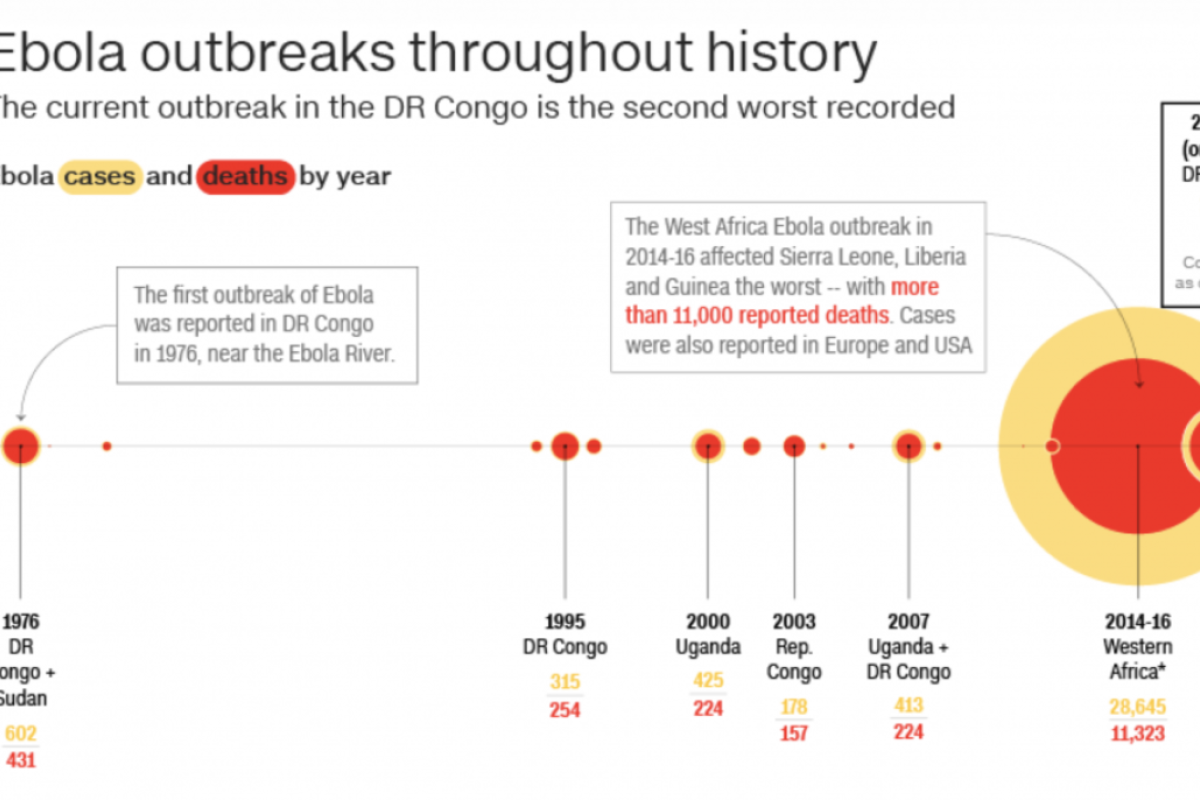
The Ebola epidemic forever changed the way WHO would handle future epidemics. At the height of the epidemic, ZMapp was rolled out for "compassionate use" which allows for the use of an unlicensed drug as a last resort when no other options are available, but only with governmental authorization. Because ZMapp was still in development at the height of the epidemic, the handful of experimental doses quickly ran out. All told, the West African epidemic of 2014-16 affected 28,616 people, mainly in Guinea, Liberia and Sierra Leone. With no hope or cure available, about 11,310 people died during the largest Ebola outbreak ever witnessed.
An Ebola clinical trial using newly manufactured ZMapp and other antidotes was started near the end of the West African Ebola outbreak, but it failed to reach a definite result before the outbreak ended. Without incidence of disease, there was no way to test drug efficacy. But, U.S. governmental agencies began to stockpile ZMapp in case the disease re-emerged.
“The re-emergence of Ebola in West Africa, and more recently in the ongoing epidemic in the Democratic Republic of the Congo highlights the need for constant vigilance in global public health. Earlier detection saves lives by mobilizing quarantine and treatment responses," Poste said. "For many emerging infectious diseases, no treatments are available or are rendered ineffective by the development of drug resistance. At least for Ebola, these clinical trials provide a much-needed treatment. The next challenge will be to monitor if Ebola develops resistance to the monoclonal antibodies in the same way that the world at large is now facing a growing problem with bacterial resistance to antibiotics."
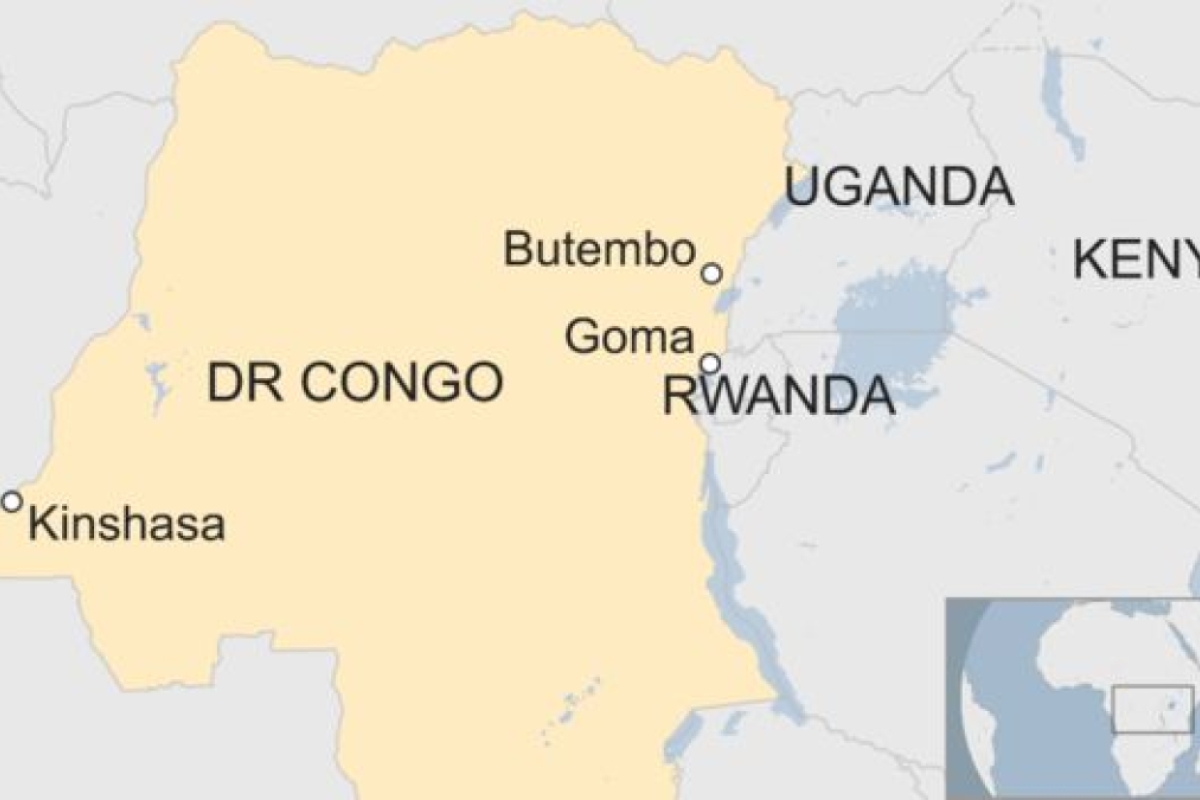
An outbreak in eastern DRC began in August 2018 and is the second-largest of the 10 to hit the country since 1976, when Ebola was first discovered. What made the latest outbreak more alarming was news that Ebola, for the first time, was spreading to big city populations.
This time, the world is much better prepared for the Ebola virus. There has been remarkable progress made toward developing an Ebola vaccine, which has been shown to be 99% effective. The first vaccine made by the drug company Merck was successfully deployed in Guinea in 2015.
More than 160,000 people have received it to date.
No cure after exposure
But for those who haven’t been vaccinated, until now, there was little hope and no cure after infection. Not everybody is vaccinated. The vaccine has been reserved for only those who come into direct contact with an Ebola patient and their families. And some people simply refused to take it.
According to the WHO, as of Aug. 6, 2019, a total of 2,781 Ebola cases have been reported in this most recent DRC epidemic. Of these, 1,866 people have died (with an overall fatality rate of 67%). Of the total confirmed and probable cases, 56% (1,572) were female, and 28% (791) were children younger than 18 years old.
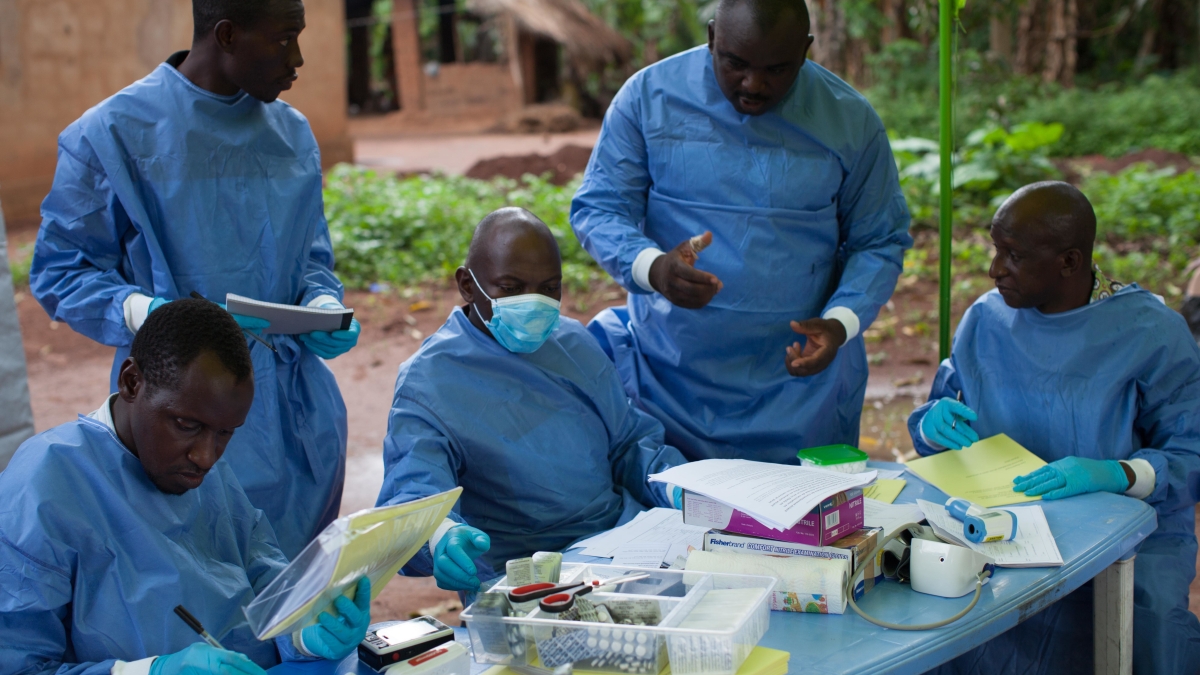
Last November, the WHO and NIH began a new clinical trial to test the newest available Ebola antidotes in the DRC. The clinical trial used ZMapp as the control drug, and compared it to three different drugs, including two newer drugs based on the ZMapp concept.
ZMapp is a cocktail made up of three monoclonal antibodies, Y-shaped proteins in the body that can recognize the specific spiky shapes on the outer shell of the Ebola virus and then recruit immune cells to attack it.
The goal of the trial was to compare ZMapp with newer formulations based on the same principle.
The drug mAb114 was developed using antibodies harvested from survivors of Ebola while REGN-EB3, like ZMapp, is a cocktail of antibodies which specifically target the Ebola virus to inactivate it. A broad-spectrum antiviral compound, remdesivir, was the fourth drug tested in the trial.
Since the start of the recent clinical trial, the four experimental drugs have been tested on around 700 patients, with the preliminary results from the first 499 now known. There were only limited and preliminary data available at this point, but they showed mortality rates of 49% in people treated with ZMapp, 53% in those who received remdesivir, 34% in people treated with mAb114, and 29% for people who received the Regeneron cocktail (compared with 2 out of 3 people, or 67%, who die if not treated).
The results were most striking for patients who received treatments soon after first becoming sick with Ebola symptoms. In this instance, death rates dropped to 11% with mAb114 and just 6% with Regeneron’s drug, compared with 24% with ZMapp and 33% with remdesivir.
As a result of the trial, the WHO and NIH agreed that all Ebola treatment units in the DRC outbreak zone will move forward, administering the two most effective monoclonal antibody drugs, the NIH’s mAB114 and Regeneron.
Does the trial spell the end of ZMapp as an Ebola therapeutic? Not necessarily. Clinical trials using naturally infected individuals in settings with minimal health care facilities are complex. U.S. government agencies are continuing their funding support of more extensive ZMapp testing, including studies to find optimal dosages of the drug.
Drug development is inherently complex, and fighting to find treatments to a disease that moves and shifts will require continued adaptation.
Will Ebola fight back?
While the world rightfully celebrated the first known cures of Ebola after infection, the scientific community knows that the Ebola threat is not over. And there are closely related viruses, such as Marburg virus, which are lurking in nature.
Because Ebola is a large RNA virus that has the ability to mutate and potentially change its response to drugs, the ZMapp and REGN-EB3 drugs were designed as a three-antibody cocktail approach. This builds redundancy into the drug, so that a viral mutation which could lead to resistance to one antibody still leaves two other parts of the cocktail to work. This design is part of the original research concept introduced by Arntzen and Mapp as an Ebola treatment strategy.
Think of it like the flu, where different strains emerge every year, which may affect people’s health differently, and the yearly flu shot formulation may be more or less effective.
With Ebola, despite the amazing success of the clinical trial, all the antidotes tested so far during the latest clinical trial are targeting only a single type of Ebola virus (the Zaire strain, which caused both the recent West African and DRC epidemics).
Mapp Biopharmaceuticals is currently designing new antibody-based drugs against the other two known strains which have caused outbreaks, the Sudan and Bundibugyo virus (there are five known species of Ebola virus) and to Marburg Virus. These efforts are building upon the original ASU-Mapp collaboration that lead to ZMapp.
And so, Arntzen has passed the baton to the next generation of ASU scientists, who are laying new groundwork.
Arntzen's longstanding ASU research team, which includes Tsafrir Mor, Hugh Mason, Qiang “Shawn” Chen and many others, have pioneered the production of pharmacologically active products in plants (principally tobacco), both to overcome health constraints in the developing world as well as the use of plant biotechnology for enhancement of food quality and value.
“Most scientists only plow in one plot,” Mor said. “Few transcend the disciplines as Charlie Arntzen did.”
Together, the team will carry on Arntzen’s quest for life saving drugs to pursue plant-based vaccines and therapeutics to combat West Nile virus, dengue fever, nerve agents and even cancer.
Their efforts have explored the possibility of plant-based anticancer agents, therapeutic agents to protect populations from bioterror threats, proteins to combat rabies, plant-derived vaccines against Hepatitis C, noroviruses and many infectious diseases.
Meanwhile, the creative folks at Mapp and elsewhere have not rested on their laurels, because they know Ebola doesn’t either. Mapp’s latest cocktail, called MBP134, is the first experimental treatment to protect animals from all known strains of the Ebola virus (formerly known as Ebola Zaire), as well as Sudan virus and Bundibugyo virus.
There hope is that it could lead to a broadly effective therapeutic, should another epidemic ever occur.
But now, there is pause for celebration, joy and greater hope than ever, as the best scientific minds around the world have in their arsenal, for the first time, new Ebola vaccines and cures.
“I am sure all of us who participated even in some small part of ZMapp development remain amazed that in only 12 years, this project could go from a hypothesis to a drug,” Arntzen said. "We are also amazed this happened with modest monetary support — only a fraction of what is normally spent on development of a new drug in the pharmaceutical industry.”
More Science and technology

ASU at the heart of the state's revitalized microelectronics industry
A stronger local economy, more reliable technology, and a future where our computers and devices do the impossible: that’s the transformation ASU is driving through its microelectronics research…

Breakthrough copper alloy achieves unprecedented high-temperature performance
A team of researchers from Arizona State University, the U.S. Army Research Laboratory, Lehigh University and Louisiana State University has developed a groundbreaking high-temperature copper alloy…

4 ASU researchers named senior members of the National Academy of Inventors
The National Academy of Inventors recently named four Arizona State University researchers as senior members to the prestigious organization.Professor Qiang Chen and associate professors Matthew…