ASU researchers develop DNA nanodevice for targeted cancer, disease treatment
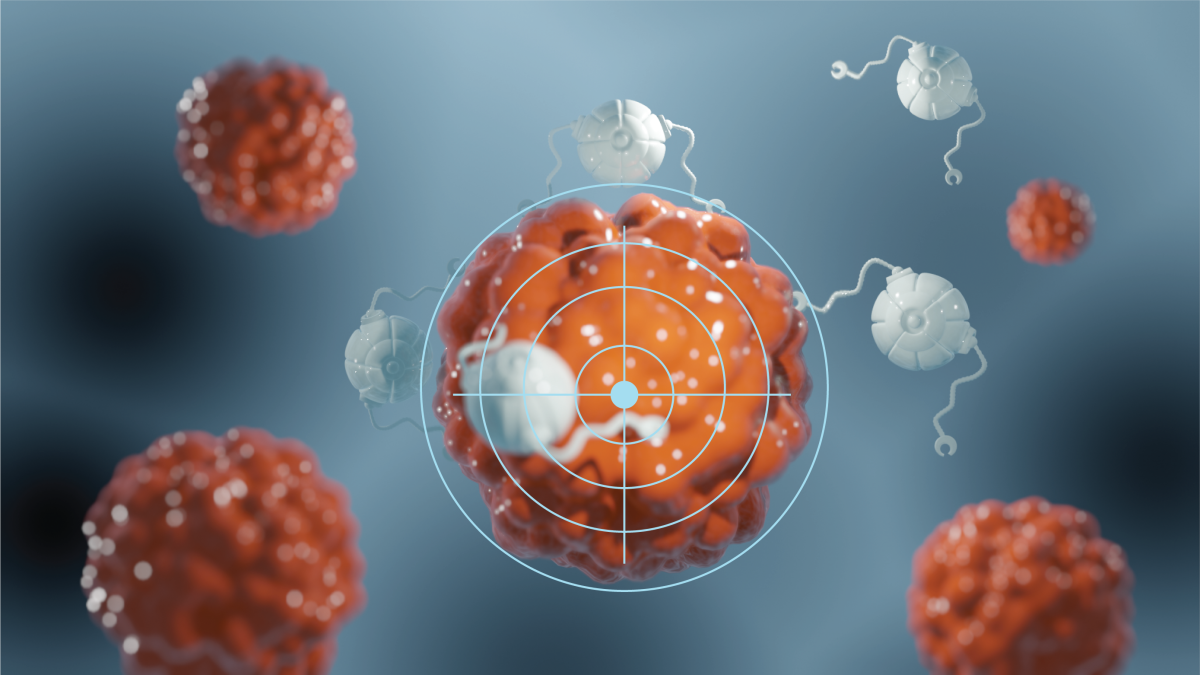
Artist's conception of a DNA nanorobot designed for targeted, deep penetration into tumor tissue. Graphic by Lu Yu
Sixty years ago, the famous physicist Richard Feynman outlined his vision for the field of nanotechnology in his landmark lecture "There is plenty of room at the bottom." Feynman foresaw new exciting applications for nanoscale devices, even conjecturing, "Although it is a very wild idea, it would be interesting in surgery if you could swallow the surgeon."
Today, that idea isn’t so wild. A research team led by professor Hao Yan from the School of Molecular Sciences and the Biodesign Institute at Arizona State University has moved the field closer toward Feynman's vision; they have developed a DNA nanodevice that can selectively target cancer cells and deliver specific intracellular treatments.
Nanotechnology has developed into a major field of research and medicine. DNA nanotechnology, a subfield that originated 40 years ago, uses designed DNA molecules as a scaffold to build nanostructures and devices through self-assembly.
Yan noted, “An important challenge in intracellular medicine is the specific targeting and efficient delivery of drugs to cytoplasm, instead of getting trapped by intracellular sorting organelles. Our approach provides a working solution to address this challenge.”
In their paper "CytoDirect: A Nucleic Acid Nanodevice for Specific and Efficient Delivery of Functional Payloads to the Cytoplasm," recently published in the Journal of the American Chemical Society, the researchers detail the development of the “CytoDirect" platform, a new DNA nanodevice capable of selectively targeting cancer cells and delivering treatment, with promising applications in intracellular medicine. This novel technology harnesses the precision of DNA nanotechnology to efficiently deliver vital medical treatments, including chemotherapy drugs and siRNAs (small interfering RNA molecules), directly into the cytoplasm of targeted cancer cells.
"One of the key innovations in this work,” explained co-author Nicholas Stephanopoulos from the ASU School of Molecular Sciences, “was using a poly(disulfide)-modified DNA strand to facilitate direct cytoplasmic delivery. Thus, the work was an elegant interplay between synthetic chemistry, DNA nanotechnology, modeling and advanced cell biology to create a functional nanodevice."
By integrating disulfide units and HER2 affibodies into a DNA origami structure, CytoDirect ensures rapid delivery to cancer cells and deep tissue penetration. The research demonstrates CytoDirect's capability to effectively transport therapeutic oligonucleotides and small molecule chemotherapy drugs, achieving notable success in gene knockdown and inducing cell apoptosis.
The development of CytoDirect is the result of collaboration and integration of experimentation and modeling utilizing several ASU facilities, including Biodesign Imaging, Flow Cytometry, Mass Spectrometry and the Life Science Electron Microscopy Laboratory.
Petr Sulc, a School of Molecular Sciences and Biodesign Institute researcher, described the importance of their multidisciplinary approach: "Just as you use computer-aided design software when you are developing new buildings, cars or computer parts, it is also of great help to be able to use computer simulations and tools to design functional nanostructures. However, it turns out that biomolecular systems are extremely difficult to model, due to the richness of the interactions they can take. At our center in Biodesign Institute at ASU, we are working closely between theory and experimental groups to develop new, efficient modeling tools to help design these nanostructures, and also make these models accessible to the wider bionanotechnology community."
Just like the "surgeon that you swallow,” this new nanodevice signifies a major leap in the use of DNA nanostructures for targeted disease treatment, offering new, promising avenues for combating cancer.
More Science and technology

ASU graduate student researching interplay between family dynamics, ADHD
The symptoms of attention deficit hyperactivity disorder (ADHD) — which include daydreaming, making careless mistakes or taking risks, having a hard time resisting temptation, difficulty getting…

Will this antibiotic work? ASU scientists develop rapid bacterial tests
Bacteria multiply at an astonishing rate, sometimes doubling in number in under four minutes. Imagine a doctor faced with a patient showing severe signs of infection. As they sift through test…

ASU researcher part of team discovering ways to fight drug-resistant bacteria
A new study published in the Science Advances journal featuring Arizona State University researchers has found vulnerabilities in certain strains of bacteria that are antibiotic resistant, just…