Designer neurons offer new hope for treatment of Parkinson's disease
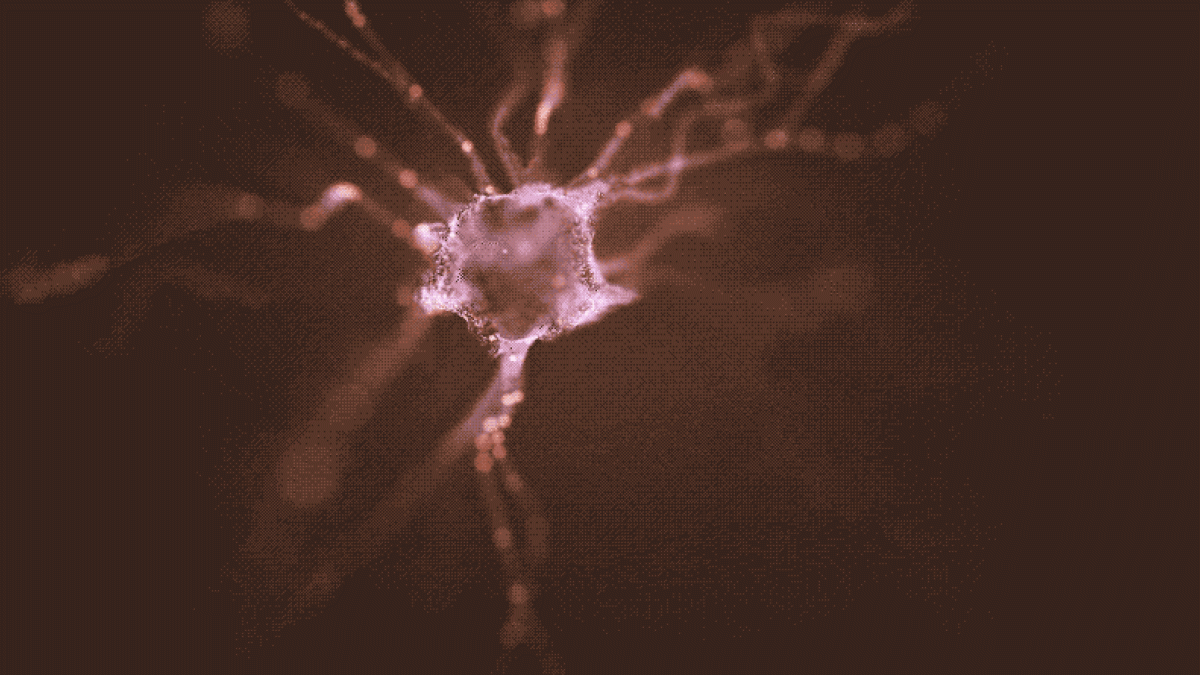
The new study describes the implantation of induced pluripotent stem cells (iPSCs) to replace dopamine-producing neurons destroyed by Parkinson's disease. Such cells not only survive the grafting procedure and manufacture dopamine, but send out their branching fibers through the neural tissue to make distant connections in the brain, just as their naturally-occurring counterparts. Illustration by Shireen Dooling
Editor’s note: This story is featured in the 2022 year in review.
Neurodegenerative diseases damage and destroy neurons, ravaging both mental and physical health. Parkinson’s disease, which affects over 10 million people worldwide, is no exception. The most obvious symptoms of Parkinson’s disease arise after the illness damages a specific class of neuron located in the midbrain. The effect is to rob the brain of dopamine — a key neurotransmitter produced by the affected neurons.
In new research, Arizona State University Professor Jeffrey Kordower and his colleagues describe a process for converting non-neuronal cells into functioning neurons able to take up residence in the brain, send out their fibrous branches across neural tissue, form synapses, dispense dopamine and restore capacities undermined by Parkinson’s destruction of dopaminergic cells.
The current proof-of-concept study reveals that one group of experimentally engineered cells performs optimally in terms of survival, growth, neural connectivity and dopamine production when implanted in the brains of rats. The study demonstrates that the result of such neural grafts is to effectively reverse motor symptoms due to Parkinson’s disease.
Stem cell replacement therapy represents a radical new strategy for the treatment of Parkinson’s and other neurodegenerative diseases. The futuristic approach will soon be put to the test in the first-of-its-kind clinical trial, in a specific population of Parkinson’s disease sufferers, bearing a mutation in the gene parkin. The trial will be conducted at various locations, including the Barrow Neurological Institute in Phoenix, with Kordower as principal investigator.
The work is supported through a grant from the Michael J. Fox Foundation.
“We cannot be more excited by the opportunity to help individuals who suffer from this genetic form of Parkinson’s disease, but the lessons learned from this trial will also directly impact patients who suffer from sporadic, or non-genetic forms of this disease,” Kordower says.
Kordower directs the ASU-Banner Neurodegenerative Disease Research Center at Arizona State University and is the Charlene and J. Orin Edson Distinguished Director at the Biodesign Institute. The new study describes in detail the experimental preparation of stem cells suitable for implantation to reverse the effects of Parkinson’s disease.
The research appears in the current issue of the npj journal Nature Regenerative Medicine.
Jeffrey Kordower directs the ASU-Banner Neurodegenerative Disease Research Center at Arizona State University and is the Charlene and J. Orin Edson Distinguished Director at the Biodesign Institute.
New perspectives on Parkinson’s disease
You don’t have to be a neuroscientist to identify a neuron. Such cells, with their branching arbor of axons and dendrites are instantly recognizable and look like no other cell type in the body. Through their electrical impulses, they exert meticulous control over everything from heart rate to speech. Neurons are also the repository of our hopes and anxieties, the source of our individual identity.
Degeneration and loss of dopaminergic neurons causes the physical symptoms of rigidity, tremor and postural instability, which characterize Parkinson’s disease. Additional effects of Parkinson’s disease can include depression, anxiety, memory deficit, hallucinations and dementia.
Due to an aging population, humanity is facing a mounting crisis of Parkinson’s disease cases, with numbers expected to swell to more than 14 million globally by 2040. Current therapies, which include use of the drug L-DOPA, are only able to address some of the motor symptoms of the disease and may produce serious, often intolerable side effects after 5–10 years of use.
There is no existing treatment capable of reversing Parkinson’s disease or halting its pitiless advance. Far-sighted innovations to address this pending emergency are desperately needed.
A (pluri) potent weapon against Parkinson’s
Despite the intuitive appeal of simply replacing dead or damaged cells to treat neurodegenerative disease, the challenges for successfully implanting viable neurons to restore function are formidable. Many technical hurdles had to be overcome before researchers, including Kordower, could begin achieving positive results, using a class of cells known as stem cells.
The interest in stem cells as an attractive therapy for a range of diseases rapidly gained momentum after 2012, when John B. Gurdon and Shinya Yamanaka shared the Nobel Prize for their breakthrough in stem cell research. They showed that mature cells can be reprogrammed, making them “pluripotent” — or capable of differentiating into any cell type in the body.
These pluripotent stem cells are functionally equivalent to fetal stem cells, which flourish during embryonic development, migrating to their place of residence and developing into heart, nerve, lung and other cell types, in one of the most remarkable transformations in nature.
Neural alchemy
Adult stem cells come in two varieties. One type can be found in fully developed tissues like bone marrow, liver and skin. These stem cells are few in number and generally develop into the type of cells belonging to the tissue they are derived from.
The second kind of adult stem cells (and the focus of this study) are known as induced pluripotent stem cells (iPSCs). The technique for producing the iPSCs used in the study occurs in two phases. In a way, the cells are induced to time travel, initially, in a backward and then a forward direction.
First, adult blood cells are treated with specific reprogramming factors that cause them to revert to embryonic stem cells. The second phase treats these embryonic stem cells with additional factors, causing them to differentiate into the desired target cells — dopamine-producing neurons.
“The major finding in the in the present paper is that the timing in which you give the second set of factors is critical,” Kordower says. “If you treat and culture them for 17 days, and then stop their divisions and differentiate them, that works best.”
Pitch perfect neurons
The study’s experiments included iPSCs cultured for 24 and 37 days, but those cultured for 17 days prior to their differentiation into dopaminergic neurons were markedly superior, capable of surviving in greater numbers and sending out their branches over long distances.
“That's important,” Kordower says, “because they're going to have to grow long distances in the larger human brain, and we now know that these cells are capable of doing that.”
Rats treated with the 17-day iPSCs showed remarkable recovery from the motor symptoms of Parkinson’s disease. The study further demonstrates that this effect is dose dependent. When a small number of iPSCs were grafted into the animal brain, recovery was negligible, but a large complement of cells produced more profuse neural branching and complete reversal of Parkinson’s symptoms.
The initial clinical trial will apply iPSC therapy to a group of Parkinson’s patients bearing a particular genetic mutation, known as a Parkin mutation. Such patients suffer from the typical symptoms of motor dysfunction found in general, or idiopathic, Parkinson’s, but do not suffer from cognitive decline or dementia. This cohort of patients provides an ideal testing ground for cell replacement therapy. If the treatment is effective, larger trials will follow, applying the strategy to the version of Parkinson’s affecting most patients stricken with the disease.
Further, the treatment could potentially be combined with existing therapies to treat Parkinson’s disease. Once the brain has been seeded with dopamine-producing replacement cells, lower doses of drugs like L-DOPA could be used, mitigating side effects and enhancing beneficial results.
The research sets the stage for the replacement of damaged or dead neurons with fresh cells for a broad range of devastating diseases.
“Patients with Huntington's disease or multiple system atrophy or even Alzheimer’s disease could be treated in this way for specific aspects of the disease process,” Kordower says.
More Science and technology

Cracking the code of online computer science clubs
Experts believe that involvement in college clubs and organizations increases student retention and helps learners build valuable…
Consortium for Science, Policy & Outcomes celebrates 25 years
For Arizona State University's Consortium for Science, Policy & Outcomes (CSPO), recognizing the past is just as important as…

Hacking satellites to fix our oceans and shoot for the stars
By Preesha KumarFrom memory foam mattresses to the camera and GPS navigation on our phones, technology that was developed for…
