It’s a tale told time and time again.
Throughout the extraordinary advancements and achievements of human civilizations, the better that we can manipulate new materials, the better our lives have gotten. Think of ancient epochs names for materials, such as the Stone Age or the Bronze Age — all the way through to the plastics and silicon fueling the digital revolution of our current age.
But what if the next tale had its origins right here at Arizona State University?
It could, if one of the co-inventors of a new field has their final say. The field — reticular chemistry — invented in the 1990s at ASU, is now sparking a slew of discoveries to solve major problems around the world today. If truly successful, the 21st century may very well become known as the "Reticular Age."
Recently, the co-inventors of reticular chemistry gathered to recall the events that led to their achievements.
The main event
The occasion for the gathering was the inaugural School of Molecular Sciences Michael O’Keeffe Lecture Series.
And no one was more fitting to kick off the lecture series than former ASU faculty member Professor Omar Yaghi (now at UC Berkeley), who together with ASU Professor Emeritus Michael O’Keeffe made their 1990s breakthrough discoveries on reticular chemistry — from humble beginnings in a Goldwater Building basement lab at ASU.
“This is an event we’ve been trying to put on for over a year,” said lecture emcee Ian Gould, a President's Professor in the School of Molecular Sciences at ASU.
ASU Emeritus Professor Michael O'Keeffe (left) catches up with ASU Regents Professor Peter Buseck.
“The purpose of this was to introduce a new event for the school. This event I believe is an important one, both for the scientific culture of the school and also to recognize the contributions of one of its most important members. Mike is one of the best but most humble scientists I think I’ve met. Based on their groundbreaking discoveries, the true excitement is that society is just now beginning to see the innovative applications based on their work,” Buseck said.
For example, their successes could pave the way to move from a fossil fuel-driven economy to a hydrogen economy by the more efficient storage of gases, help us harvest more water for a thirsty world, or even remove excess carbon from Earth's atmosphere.
“It’s a real pleasure to be here on many, many levels, not the least of which is to speak on behalf of Michael O’Keeffe and my collaborations with him in inventing a new branch of chemistry,” said Yaghi, who gave his first in-person lecture since the pandemic, titled “Reticular Chemistry and Materials for Water Harvesting from Air Anytime, Anywhere.”
Over the course of the lecture, Yaghi traced back the key moments of discovery, and the past, present and future of the reticular chemistry field he and O’Keeffe helped establish.
A reception put on by the School of Molecular Sciences honored Michael O'Keeffe and kicked off an inaugural lecture series in his honor. Photo courtesy of Amy Chow
A homegrown achievement for ASU
O’Keeffe, now an 87-year-old professor emeritus who remains active in his field, spent his entire academic career at ASU. In 1963, he was hired by Leroy Eyring in what was then known as the Department of Chemistry. O’Keeffe quickly gained a reputation as a relentless and highly creative innovator.
“We have chemical royalty in the person of Michael O’Keeffe,” said longtime colleague and friend Peter Buseck, a Regents Professor in ASU's School of Molecular Sciences and School of Earth and Space Exploration, who was hired the same year as O’Keeffe. “He exemplifies what one can do at ASU. There have been astounding accomplishments here.”
To foster innovation, new materials are always in demand as the vital building blocks behind any new technology. But according to O’Keeffe, “although the synthesis of new materials has long been recognized as the most essential element in advancing technology, it generally remains more of an art than a science — the discovery of new compounds has mostly been serendipitous, using methods referred to by critics as “shake and bake,” “mix and wait” or “heat and beat.”
ASU Emeritus Professor Michael O'Keeffe
O’Keeffe’s background and expertise are on the crystallography and theoretical geometries of chemical crystals. For example, ordinary table salt can be formed into cube-shaped crystals of alternating sodium and chloride atoms. O’Keeffe combined an architectural mindset to his way of conjuring up new materials in 2D and 3D space.
Joining him at ASU in 1992 was Yaghi, a young, ambitious wizard in chemical synthesis and growing crystals. Twenty years ago, they embarked on a quest together to upend the limits of chemistry at the time.
“When I joined ASU, I was only 26 years old,” Yaghi said. At the time, he said he thought: “I have to get tenure and make a difference.” He sat down in O’Keeffe’s office in the Physical Sciences Building of what was then the Department of Chemistry and Biochemistry.
“I went to his office to brag about my new result,” Yaghi recalled.
The reserved, U.K.-native O'Keeffe took the occasion to challenge Yaghi to see if he could synthesize a particularly beautiful, complex crystal model in his lab.
“'I bet you can’t make that,'” Yaghi said O’Keeffe told him.
Yaghi brashly replied, “No, I bet you that I can make it!”
Professor Omar Yaghi
And so, Yaghi, along with his graduate students, worked around the clock in his lab in the basement of Goldwater lab. “I took O’Keeffe’s model and gave it to my graduate student.”
Thus began the field now known as reticular chemistry. They made a porous metal sulfide — a metal organic framework, or MOF — unlike any other.
“This was a whole new field of research, right here,” Yaghi said.
Yet there were many doubters at the beginning.
O’Keeffe explained, “Those who went to conferences where such materials were discussed two decades ago heard the chorus: 'They won’t be stable.' They were. 'The frameworks will collapse when solvent is removed.’ They didn’t. 'They won’t be porous.' They were — they adsorbedAdsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. Source: Wikipedia. gases at low pressures and had 'permanent' porosity."
Follow the ASU yellow ball
O’Keeffe typically filled in his models with a placeholder of a yellow balloon to show the size of a molecule that could fit within the space.
This porosity — a big opening in the heart of a MOF structure — O’Keeffe typically filled in his models with a placeholder of a yellow balloon to show the size of a molecule that could fit within the space. His yellow, space-filling balloons became a hallmark of their MOF designs.
“He put the yellow ball in there, which has become famous since then,” Yaghi said. “I made the decision that the yellow ball should be yellow because that was Arizona State University’s logo.”
They invented a new crystal chemistry of stable and porous MOFs. They came up with all sorts of different sizes that they could design and fill with an assortment of molecules placed into spaces within these structures.
An early example of an MOF, formed of strong bonds, was what is now known as MOF-2, made by Yaghi’s lab team. In this compound, square "paddle wheels" containing two zinc atoms are linked in a periodic square array. It was one of the first structures that had the ability to adsorb gases within the big spaces at low pressures.
O’Keeffe said of their discovery, “What has been most remarkable in MOF chemistry over the years was the constant Greek chorus saying that ‘It can’t be done,’ but subsequent history has been an illustration of the truism ‘never say never' — naysayers can only be proved wrong.”
And soon, they did just that. One structure in particular, MOF-5, attracted worldwide attention for its unparalleled ability to hold gases. It earned O’Keeffe, Yaghi and colleagues a seminal publication in the prestigious journal Nature. It changed everything.
“And so, we were off to the races,” Yaghi said.
This demonstrated to the scientific community that MOFs had truly unprecedented surface area, porosity and stability.
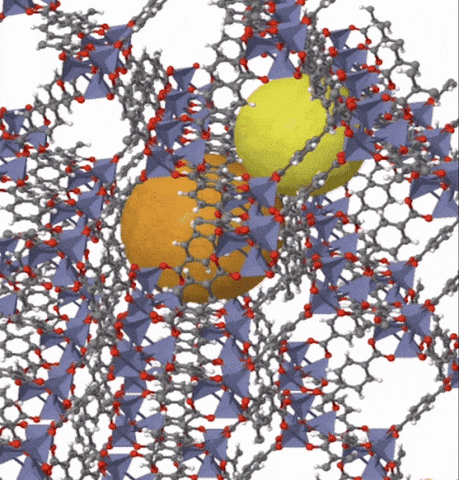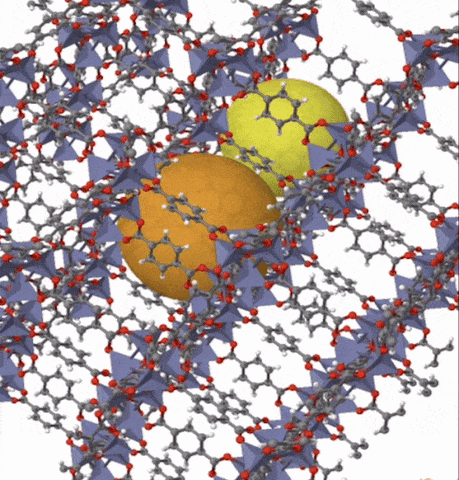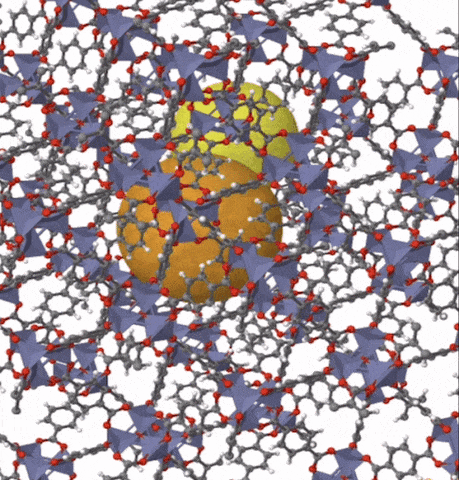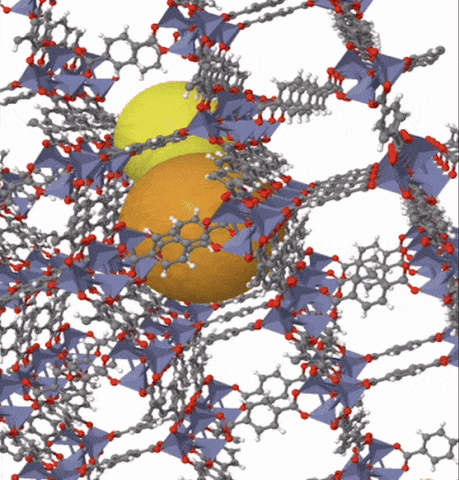
Above: MOF-5 (sometimes called IRMOF-1) is a metal organic framework formed from Zn4O nodes with 1,4-benzodicarboxylic acid struts between the nodes. The spheres represent the pore size that can be used for gas storage. IR stands for isoreticular which means it is a series of MOFs with the same topology, but different sized pores.
“We had to show, in fact, that you could make an MOF, not just crystalline, but that the pores can remain open in the absence of guests,” Yaghi said. “And everyone was excited.”
They took from the fields of organic, inorganic and solid-state chemistry, and combined this know-how with the art and aesthetics of architecture. Together, from a toolkit of molecular Legos, they fashioned chemical building blocks into entirely new frameworks, with exotic, 3D crystals built in new triangular, square and tetrahedral geometries.
These MOFs were precisely arranged, with strong bonds containing atoms of carbon, hydrogen and oxygen combined with metals like zinc for entirely new properties, like storing gases more compactly.
For the first time, scientists could use the pores for new applications. That was the aspect that was missing from the field.
“It gave this field, (which) I would say was full of sculptures ... a basis to go on and make frameworks that are now useful,” Yaghi said. “Now you can remove the gas and put new ones in. The new ones could be hydrogen, methane, whatever. Or you could functionalize the pores and do very specific transformations. So, this meant everything.”
An illustration shows the type of geometries that can be made by metal organic frameworks, or MOFs, which can be made with strong bonds containing atoms of carbon, hydrogen and oxygen combined with metals like zinc for entirely new properties, like storing gases more compactly. Illustration courtesy of Alex Cabrera
A beautiful collaboration
“Omar, this is so beautiful! It will be the best thing you’ve ever done!” O’Keeffe said at the time.
“Little did he realize that we would do much more than that together,” Yaghi said.
“That really was the magic of that collaboration. It didn’t take Mike very long to recognize that basically any building unit that you could get your hands on (that) could be a geometric unit could be linked together into a network. What this meant was that any linker, any cluster that you can imagine you can get your hands on, you can make it into an MOF. Anything a chemist can imagine, now, we’ve shown that you can make,” Yaghi said.
They found that only a small number of structures would be of overriding importance. Quickly, them came up with a new periodic table of reticular chemistry. They started with basic shapes: triangles, squares and branches. Like tinker toys, these different geometries allowed them to progressively build with higher connectivity and higher complexity.
And unlike the polymer chemists of the 20th century who built "chain builders" of plastics like nylon or polyester, the field of reticular chemistry was wide open. Chemists could now, for the first time, build in 2D or 3D dimensions. Soon, MOFs begat covalent organic frameworks (COFs), which used carbon as linkers to make very strong bonds that were stable — even in acid.
“That is the holy grail of COFs,” Yaghi said. “The rest is trial and error, and the path is already open.”
A brand-new framework
Yaghi thinks chemists can now leap from the molecular age — the control of matter in zero dimensions — to the reticular age: the control of matter by building on the molecular level in all dimensions.
“A chemist can twist around an atom in a molecule (and go) from being a poison to a medicine,” explains Yaghi in a YouTube video. “It’s that type of control that you want to have on this hidden world to allow you to do beneficial things.”
Yaghi hopes reticular chemistry can make a big difference on three major stressors facing the planet today: clean fuels, clean air and clean water.
“A child today breathes in twice the amount of carbon dioxide as before the industrial revolution,” Yaghi said.
The first practical applications based on MOF technology include automobile gas tanks to store natural gas, or down the road, hydrogen gas, which would enable a hydrogen-powered economy. “We can stack hydrogen like cars in a car park,” Yaghi said.
Typically, gases like to repel each other. Like storage containers, the porous MOFs can be used like sponges to hold gas molecules, and then be stacked on top of the other to make more efficient use of space. There is a lot of interest because MOFs are easy to make, and they can be scaled up to make in multi-ton quantities.
A schematic shows a new device that can harvest water from the air.
MOFs can also be used as a clever new material to capture carbon from the air and remove harmful carbon dioxide from the atmosphere.
More recently, MOFs have even been used to harvest pure water from the air.
“We think we can harvest at even at low humidity,” Yaghi said. “When you can harvest water from desert air, you can do it anywhere, at any time of the year. One-third of the world is currently experiencing water stress, and there are always questions of water purity. We can hopefully help the world achieve water independence.”
A generational impact to change the world
From their start at ASU, O'Keefe and Yaghi have touched and inspired the next generation of scientists around the world.
“From that 1995 publication, there are now groups in 102 countries around the world doing MOF research,” Yaghi said.
From their start at ASU, the research duo have touched and inspired the next generation of scientists around the world.
Today, scientists are forming startup companies to explore commercial applications, or trying to incorporate 3D printer manufacturing technologies for MOFs.
Since their first discoveries, O’Keeffe and Yaghi have both ranked within the top five most-cited chemists in the world during the past decade, showing their academic impact among the scientific community. According to the top 100 chemists, ASU is the third-ranked institution with the six most influential chemists between 2000 and 2010, with Yaghi and O’Keeffe ranked in the top three.
In honor of their achievements, O’Keeffe and Yaghi were awarded the Aminoff Prize in 2018 from the Royal Swedish Academy of Sciences.
Based on their ingenuity, there are now thousands of MOF structures that have been produced, each with a new variation on their classic themes. O’Keeffe and Yaghi have kept a worldwide searchable database containing more than 100,000 of these structures.
They have established a systematic design and understanding of MOF structures, and the basis of a whole new chemistry.
“There has been nothing like it in the history of chemistry,” O’Keeffe said.
And despite having emeritus status, O’Keeffe continues to perform research and has many new discoveries and papers in the works. More recently, O’Keeffe became very interested in nets and weavings, and the symmetry, design and computation for chemical crystal nets, with ASU collaborator and physics Professor Mike Treacy.
At the end of the lecture, Yaghi reflected on his interactions with O’Keeffe throughout the decades, saying, “I think it’s been a wonderful journey, and we will continue on that course. And I hope this tradition will continue.”
Top photo: ASU Professor Emeritus Michael O’Keeffe and UC Berkeley Professor Omar Yaghi.
More Science and technology

Lessons on maintaining your humanity in the world of AI technology
AI is not human. But it does a good job of acting like it.It is capable of replicating how we speak, how we write and even how we solve problems.So it’s easy to see why many consider it a threat, or…

When you’re happy, your dog might look sad
When people are feeling happy, they’re more likely to see other people as happy. If they’re feeling down, they tend to view other people as sad. But when dealing with dogs, this well-established…

New research by ASU paleoanthropologists: 2 ancient human ancestors were neighbors
In 2009, scientists found eight bones from the foot of an ancient human ancestor within layers of million-year-old sediment in the Afar Rift in Ethiopia. The team, led by Arizona State University…








