Protein structural insights chart the way to improved treatments for heart disease

Assistant Professor Wei Liu from ASU’s School of Molecular Sciences and the Biodesign Institute’s Center for Applied Structural Discovery. Photo credit Mary Zhu
A team including Wei Liu, assistant professor in ASU’s School of Molecular Sciences and the Biodesign Institute’s Center for Applied Structural Discovery, published a paper on Aug. 19 in Molecular Cell that offers promising details of improved therapeutic treatments for cardiac disease.
Cardiac disease is the No. 1 killer of people worldwide and according to the U.S. Centers for Disease Control, it kills one person every 37 seconds in the United States.
With this in mind, the teamThe team included Lan Zhu, Ming-Yue Lee, Dewight Williams and Wei Liu from ASU; Minfei Su, Raja Dey, Jianyun Huang, Joel R. Meyerson and Xin-Yun Huang from Cornell University; Yixiao Zhang and Thomas Walz from The Rockefeller University; as well as Navid Paknejad and Richard K. Hite from Memorial Sloan Kettering Cancer Center; Kelsey D. Jordan and Edward T. Eng from New York Structural Biology Center; and Oliver P. Ernst from the University of Toronto. decided to conduct structural and functional studies using cryo-electron microscopy (EM) to capture never-before-seen detailed conformational changes involving the β1-adrenergic receptor (β1-AR) in complex with the Gs protein. The β1-adrenergic receptor is a member of the G protein-coupled receptor (GPCR) family. GPCRs are the largest class of membrane proteins in the human genome.
β1-ARs are predominantly expressed in the adult human heart and dominates as a major regulator of cardiac function. The activated receptor triggers Gs-protein coupling and increased cardiac 3′-5′-cyclic adenosine monophosphate (or cAMP for short) levels. These molecular events manifest physiologically as increased heart rate, increased conduction, reduced refractoriness within the atrioventricular node, increased contractility, and increased cardiac output.
Downregulation of β1-ARs has been seen as the cause of most cases of heart failure, one of the leading causes of morbidity worldwide. Beta-blockers, which are inhibitors of β1-ARs, are used to treat high blood pressure and heart failure, to manage abnormal heart rhythms, and to protect against myocardial infarction.
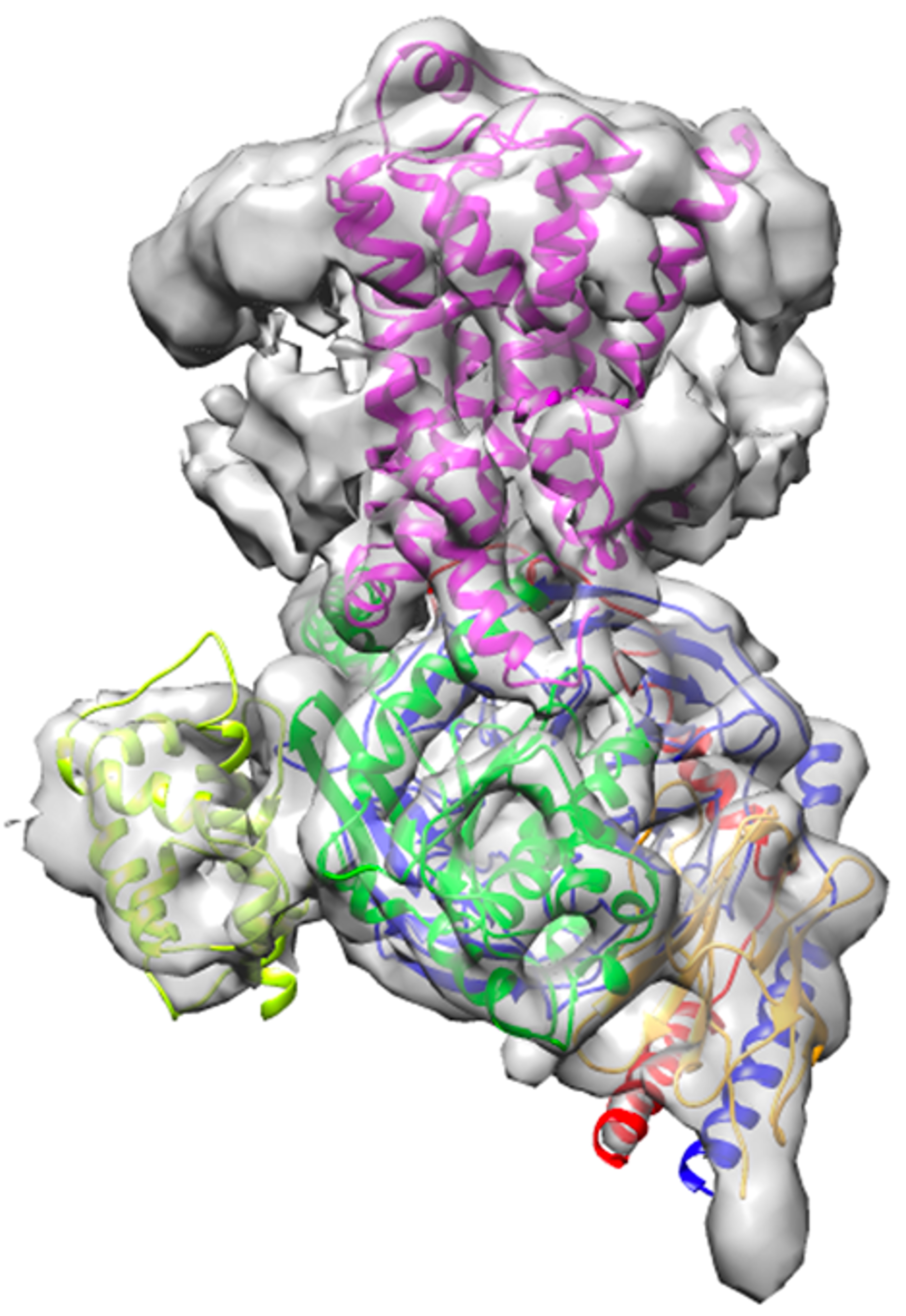
Overall architecture of isoproterenol-bound β1-adrenergic receptor and Gs complex cryo-EM density map. The 2.6 Å high-resolution structure offers novel insights of this important drug target.
“In this Molecular Cell paper, we employed cryo-electron microscopy and signaling studies to investigate the molecular mechanism by which β1-AR catalyzes the guanine-nucleotide exchange as the result of Gs activation” said Liu.
“We have captured never-before-seen details of the conformational changes during the Gs activation by isoproterenol-bound β1-AR. Activated β1-AR, serving as a guanine-nucleotide exchange factor (GEF) for Gs, deforms the GDP-binding pocket and induces a tilting of the C-terminal α5-helix and the α-helical domain of Gs rotational opening away from its Ras-like domain,” explained Lan Zhu, assistant research scientist in the School of Molecular Sciences and Biodesign Center for Applied Structural Discovery and one of four co-first authors of this paper.
The other first authors include Minfei Su of Cornell University, Yixiao Zhang of The Rockefeller University and Navid Paknejad of Memorial Sloan Kettering Cancer Center.
"This structure of the adrenergic receptor complex with the effector G-protein reveals molecular details in the protein-protein interaction domains involved in the receptor activation,” said Liu. “This information allows for the design of new precision therapeutics to target cardiac diseases, one of the leading causes of death in the developed world."
In the past few years, single-particle cryogenic electron microscopy (cryo-EM) in particular has triggered a revolution in structural biology and has become a newly dominant discipline. Cryo-EM allows researchers to take a look at biological structures that were simply not accessible just a few years ago and is now exposing structures of unprecedented complexity in great detail.
Lan Zhu
Indeed, it is this technique utilized by the experts in the School of Molecular Sciences and the John M. Cowley Center for High Resolution Electron Microscopy in the College of Liberal Arts and Sciences at ASU that has enabled the current research.
“Wei Liu’s work is typified by outstanding scholarship and a relentless commitment to making critical advances that will benefit science and society at large,” said Ian Gould, interim director of the School of Molecular Sciences.
These new results provide structural insights into the activation mechanism of Gs by β1-AR and offer extremely promising details for improved therapeutic treatments for cardiac disease.
More Science and technology

Study reveals genetic insight into desert survival
The deserts of the American Southwest are home to the Mojave and Sonoran desert tortoises, two seemingly similar yet genetically…

Study reveals lasting effects of common weed killer on brain health
Environmental exposure to toxins in the air, water or certain chemicals can increase the risk of ill health effects, including to…

ASU grad to use science to get an edge on crime
Editor’s note: This story is part of a series of profiles of notable fall 2024 graduates.As a child growing up in Pinetop,…