ASU professor details hazardous materials risks in incidents like Tempe Lake bridge derailment

A 95-car, Union Pacific freight train derailed over Tempe Town Lake on Wednesday, July 29. Three cars landed in the empty park below the south end of the bridge, two of which contained the toxic chemical cyclohexanone.
Initially, there was minor leakage into the ground from one of the derailed cars. On Saturday, Aug. 1, two workers were splashed with the chemical while transferring the contents of the downed cars to an alternative vehicle before removing the damaged tanks.
Kiril Hristovski, an associate professor in Arizona State University's Polytechnic School, is the program chair for the Environmental and Resource Management Program with expertise in hazardous materials management. He gave ASU Now an overview about the transportation of hazardous materials on railways and the handling of toxic materials, especially the cyclohexanone released in the Tempe incident.
MORE: For information about the structural components of the bridge, see ASU engineers offer insight on Tempe railway bridge collapse.
“Knowledge and training are the most important tools that anyone who deals with hazardous materials should have,” Hristovski said. “This is why many of the regulatory requirements focus on them as part of the prevention and mitigation approaches.
“The emergency responders in Tempe showed exceptional professionalism and did an outstanding job preventing a bad scenario of becoming a worse.”
Question: In the U.S., how frequent are railroad incidents that involve hazardous materials like the one that occurred in Tempe?
Answer: According to the U.S. Department of Transportation, the number of railroad incidents that involve hazardous materials in the U.S. has been generally decreasing over the last decade, from about 750 in 2010 to about 570 in 2017, and 500 in 2018.
In 2019, the DOT reported the lowest number of railroad incidents involving hazardous materials — 420, which represents less than 5% of the total number of incidents. For example, in 2019, fewer than 2% of all incidents during transport of hazardous materials were railroad incidents.
In Arizona, the number of incidents also has been decreasing over the last five years. In 2016, there were 13 incidents, while these numbers dropped to nine and five in 2017 and 2018, respectively. In 2019, there were only three railroad incidents related to hazardous materials.
Seven months into 2020, we already have reached this number of incidents.
The total number of fatalities for these accidents in the U.S. has been one person in 10 years, which is significantly lower than the highway transportation. Over the last 10 years, we have about 100 highway fatalities.
Although the railroad hazardous materials release incidents are not as frequent as highway incidents, they can constitute up to 40% or more of the total damages in the U.S.
In 2015, the damages related to railroad hazmat incidents were about $46 million. However, we see this downward trend over the last five years. In 2019, the costs associated with these damages dropped to about $17.5 million.
This indicates that there are good trends over the last decade in terms of preventing and minimizing hazardous material-related railroad accidents.
Q: What are some of the dangers of railroad incidents where chemicals, like cyclohexanone, are released from derailed railcars?
A: Cyclohexanone and many similar organics are flammable liquids. The greatest hazard from such liquids is their flammability. This comes from a lower explosive limit in air, which is 1% by volume.
This means that if we have 1% by volume of cyclohexanone vapors in air, and we have a spark, it can ignite, or catch on fire. It is very easy to cross this 1% limit for cyclohexanone, because it can start boiling at about 270 degrees Fahrenheit and before it reaches this temperature, it can provide a sufficient amount to vapors to create a flammable mixture with air.
At 111 degrees Fahrenheit, we have enough cyclohexanone vapors in the air that they can support a fire with the addition of a single spark.
The National Fire Prevention Association classifies cyclohexanone as a Class II combustible liquid and the DOT classifies it as Class 3, Flammable. In order to get enough vapors for it to burn, it must be heated to above 100 degrees Fahrenheit.
In Arizona in the summer, the temperature outside is frequently over 100 degrees Fahrenheit in the shade, so for this chemical in the Arizona summer, there’s already a heat threshold that can create enough vapors to support burning. We just need a spark.
These conditions are what made the Tempe Union Pacific railroad incident so dangerous, especially because there was fire involved in the derailment.
The emergency responders — fire and police — did a very good job of reducing the dangers in a timely manner, especially in the heat. They prevented potentially devastating scenarios considering the amount of flammable liquid and the ongoing leaks. So one can easily say that they were heroes who saved the day.
If they had been unable to contain the fire and the leaking cyclohexanone tank had caught on fire, there would have been a new level of hazard possibilities.
In the case of a fire, an incomplete combustion of cyclohexanone can create heavy smoke, which contains carbon monoxide (CO) — an extremely toxic gas. The smoke potentially contains many carcinogenic compounds and different tiny organic particles.
The smoke can cover a large area, presenting dangers to those in a densely populated area like that of the Tempe bridge incident.
When dealing with fires involving organic compounds in closed tanks, one of the worst things that can happen is a boiling liquid expanding vapors explosion, known as BLEVE. As a result of a strong fire or damage, the structural integrity of a tank, like a railcar tank that is partially filled with an organic liquid, can be diminished. The heat from the surrounding fire will cause the liquid in the tank to boil, creating high pressures. These pressures can cause rupture of the tank, which will rapidly release the pressurized vapors. These vapors can easily ignite and propel the tank dozens, if not hundreds, of yards, resulting in a huge fiery projectile flying in the air.
Such a scenario happened in Arizona on July 5, 1973, on Interstate 40 when a BLEVE incident involving a propane railcar killed 11 emergency responders and a number of spectators. The casualty report was 107 people injured or killed.
The level of complexity of hazmat-related railroad incidents can easily increase if other chemicals are involved in a scenario. Some incidents may involve release of toxic gases, corrosive materials, highly reactive chemicals … the hazards can be diverse. They can affect people, property, and the local environment.
Q: Is cyclohexanone toxic?
A: From a toxicity perspective, cyclohexanone is not classified as a human carcinogen or mutagen. It is a serious skin irritant that can cause severe eye burns if splashed in the eyes. It can be harmful if ingested or inhaled. It can cause respiratory irritations if a person is exposed to it. The recommended 15-minute, short-term exposure limit is 50 ppm, which can be easily exceeded, so proper personal protection equipment is required for handling.
From an ecotoxicity perspective, cyclohexanone is considered toxic to aquatic organisms and may cause adverse effects in the aquatic environment. Unlike many organic chemicals, which are immiscible, or unable to mix with water, cyclohexanone is soluble in water to about 150 grams per liter. However, it is readily biodegradable, does not accumulate and it is not classified as marine pollutant.
Reports suggest that more than 75% of an initial spilled amount can be degraded within 4 weeks, although this standard is for climates that are not as hot as Arizona. Here, most of the cyclohexanone will likely evaporate more because of its relatively high vapor pressure and high temperatures — unless it enters drains.
Cyclohexanone shares similar hazards with many crude oil derivatives where the primary hazard is typically flammability. However, many oil derivatives are water immiscible so they can be more difficult to fight in case of a fire because the majority of them will tend to float on the water, so water may not be the best option to extinguish such fires. Emergency response professionals in the U.S. are trained for such scenarios.
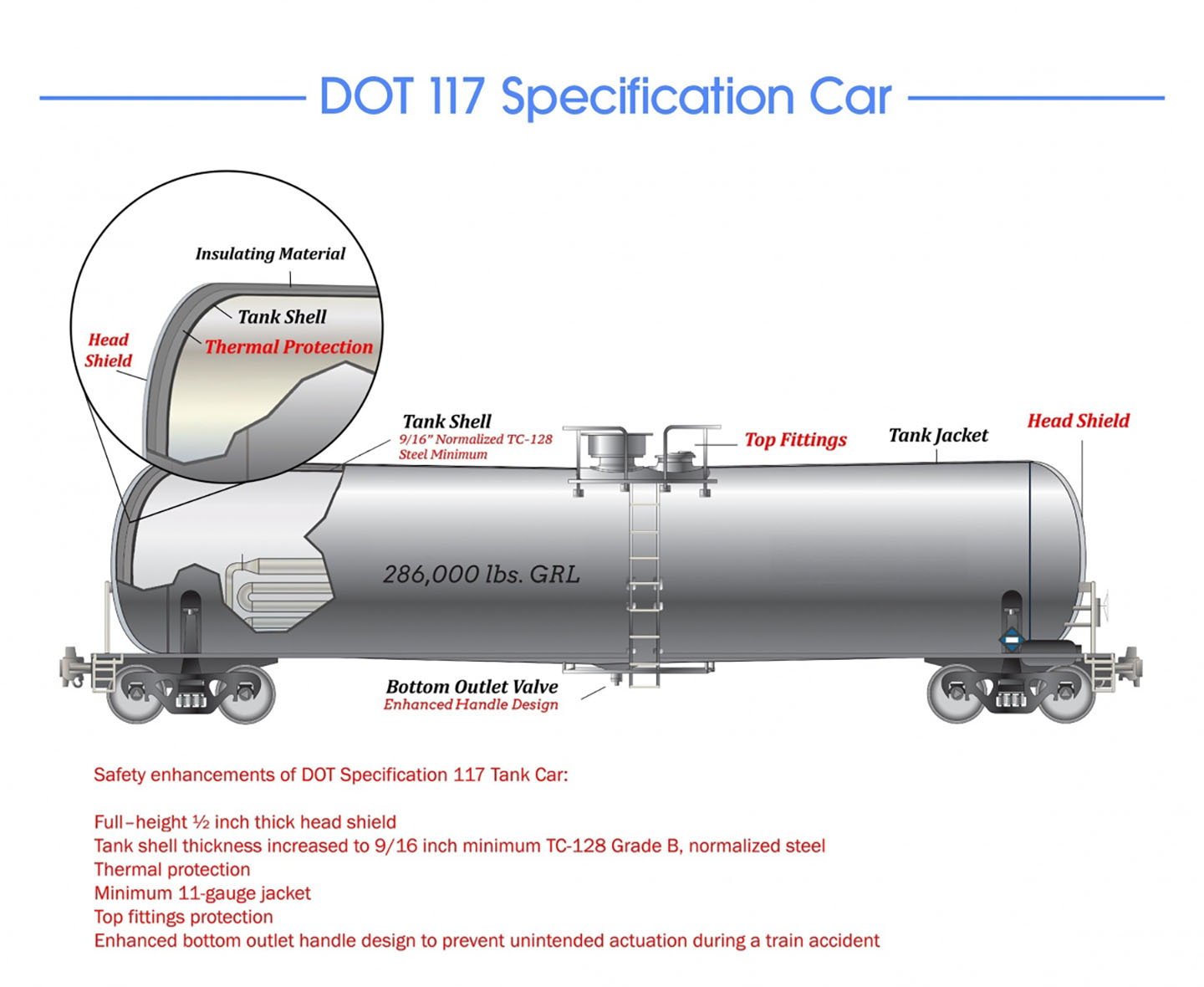
U.S Department of Transportation illustration of a tank car commonly used to transport flammable liquids like cyclohexanone.
Q: When there is a train derailment with a chemical release, like cyclohexanone, what is typically done to minimize the adverse effects and protect the public, property, and the environment?
A: While derailment incidents in privately-owned railroad bridges are the domain of the Federal Railroad Administration (FRA), the DOT has the primary jurisdiction in regulating transport of hazardous materials when they are moved by any means of transportation in the U.S., including railroad. The DOT has an entire set of regulations and standards that regulate almost all aspects of transportation, including preparation and packing of the hazardous materials for transport to the delivery.
Almost all of these regulations and standards are promulgated to prevent and reduce the number of incidents involving hazardous materials and minimize the effects in case they happen. For example, DOT has specific standards for how the railcars have to be constructed for different hazardous materials. These can be found in the Code of Federal Regulations, 49 CFR 179.
The design and performance requirements for the DOT-117 railway tank car, which is commonly used to transport flammable liquids, include safety features like head shields to minimize damage in case of derailment, shells made of thicker materials, like thicker steel, insulating materials, thermal protection, specialty valves, and so on. The older DOT-111 railway tank cars did not have many of these safety features.
Furthermore, special placarding requirements are in place, which can tell first responders from a distance what is in the railcar tanks, identified by a United Nations or North American number that coincides with a specific hazardous chemical, and whether there is immediate danger. Responders use this information to quickly obtain information about possible hazards and how to respond to isolate the incident, evacuate the public and what to do in unique scenarios. There are specific, standardized procedures in place so all of the activities can be quickly synchronized.
In hazmat scenarios, as the time passes after an incident, the higher the probability that the situation can get worse. The placards help first responders to immediately assess the hazards, select appropriate responses, and begin containment process when necessary.
For the response to be effective, extensive training is required for everyone who is involved in any aspect of working with hazardous materials.
To illustrate, when an emergency or a first responder sees the placard with a DOT hazard class and the chemical hazard number, they can access almost all the necessary hazard and response related information within seconds. The shipping papers, which are typically held in the possession of the train engineer or the conductor, have more information, but it frequently takes valuable time to access those documents.
In the case of cyclohexanone, there is a red diamond with a flame sign on it that says Flammable 3 and the number 1915. This number tells the first responder that the car carries cyclohexanone, allowing immediate access to information that they are dealing with a water miscible, flammable liquid whose vapors can form explosive mixtures with air; that these vapors may travel to a source of ignition and flash back; that the containers may explode when heated; and that inhalation or contact with material may severely irritate or hurt skin and eyes.
This is why the workers transferring the cyclohexanone from the damaged railcar to another transport vehicle were treated immediately when the tubing broke and the workers were contaminated.
A cyclohexaclone-related fire may produce irritating, corrosive and/or toxic gases. With a large spill, an evacuation downwind to at least 1,000 feet is required, and in a case of fire, at least half a mile.
The information from the railcar placard also helps responders quickly discern what extinguishing agents they may use and how, what measures can be used to contain the spill and what first aid measures are effective.
Top photo courtesy of H.H. via Pexels.com.
More Science and technology

Applied Materials invests in ASU to advance technology for a brighter future
For nearly 60 years, global giant Applied Materials has been hard at work engineering technology that continues to change how microchips are made.Their products power everything from flat-panel…

Meet ASU engineering students who are improving health care, computing and more
Furthering knowledge of water resource management, increasing the efficiency of manufacturing point-of-care health diagnostic tools and exploring new uses for emerging computer memory are just some…

Turning up the light: Plants, semiconductors and fuel production
What can plants and semiconductors teach us about fuel production?ASU's Gary Moore hopes to find out.With the aim of learning how to create viable alternatives to fossil-based fuels, Moore — an…

