Sun-powered nanotechnology could supply clean water and renewable energy

Researchers including Arizona State University Assistant Professor Christopher Muhich are using quantum chemistry techniques to develop a method that applies the power of the sun as a photocatalytic spark to produce a water-disinfecting chemical. Photo courtesy of Shutterstock
Hydrogen peroxide is commonly known as a household disinfectant for minor cuts and scrapes and a bleaching agent used in teeth-whitening products.
But this weak acid is also a strong oxidizing agent — a chemical compound that extracts electrons from other substances. Because of that characteristic, hydrogen peroxide could be the key to a potentially more effective method for removing contaminants from water.
Progress in the realm of quantum chemistry is producing more knowledge of the fundamental characteristics of chemical reactions, said Christopher Muhich, an assistant professor of chemical engineering in the Ira A. Fulton Schools of Engineering at Arizona State University.
The insights raise possibilities for new ways to trigger photocatalysis, the process in which light interacts with matter to drive specific kinds of reactions, Muhich said.
The research journal Proceedings of the National Academy of Sciences, or PNAS, recently published a paper by Muhich and his research collaborators detailing investigations into using sunlight as the photocatalytic spark to generate hydrogen peroxide from water and oxygen.
For the same reason hydrogen peroxide is a good antibacterial disinfectant, it is also good at degrading organic pollutants, Muhich said, and its use for water decontamination would not require either other chemicals or the large amounts of electricity typically used in traditional water treatment methods.
Such a system would also offer a solution for rural communities that have little or no access to municipal water systems, he added.
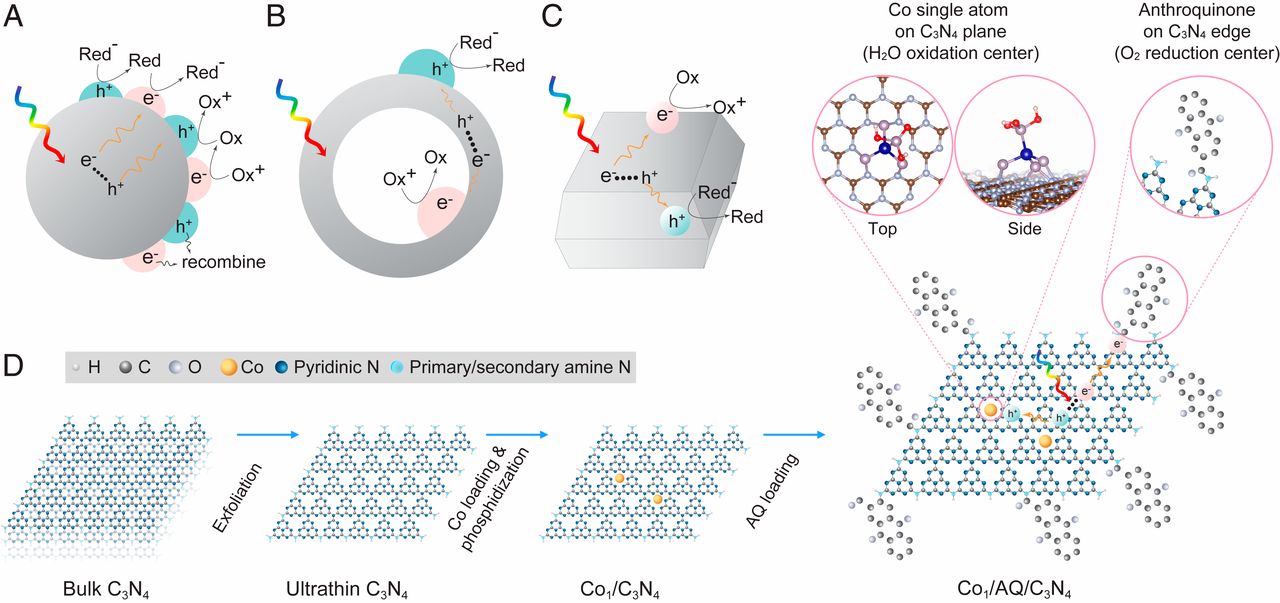
The illustration depicts the novel catalyst developed by Fulton Schools Assistant Professor Christopher Muhich and his research collaborators to combine light harvesting and catalytic materials in a way that enables energy from the sun to drive chemical reactions to produce hydrogen peroxide for disinfecting water. The research detailed in the Proceedings of the National Academy of Sciences journal was led by Professor Jaehong Kim, chair of chemical and environmental engineering at Yale University. The paper’s lead author is Chiheng Chu, a postdoctoral researcher at Yale. Scientist Eli Stavitski at the Brookhaven National Laboratory performed the spectroscopy for the project and Fulton Schools chemical engineering doctoral student Srishti Gupta performed the density functional theory calculations to understand the process. The illustration is from “Spatially separating redox centers on 2D carbon nitride with cobalt single atom for photocatalytic H2O2 production”/Proceedings of the National Academy of Sciences
Combining light harvesting and catalytic materials
Muhich is working on the photocatalysis project with scientists and engineers at Yale University and Brookhaven National Laboratory through the Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment, or NEWT, a consortium of researchers at Yale, ASU, Rice University and the University of Texas at El Paso supported by the National Science Foundation.
NEWT’s mission is to enable access to potable water by developing next-generation high-performance and easy-to-deploy drinking water and industrial wastewater treatment systems using advances in nanotechnology.
In doing so, the center employs the unique properties of engineered nanomaterials to treat water using fewer chemicals, less electricity and smaller reactors than current technologies — while also decreasing treatment costs.
Muhich and his colleagues are attempting to not only devise a new water treatment method — primarily to make water suitable for drinking — but also to use the solar energy and hydrogen peroxide system to produce renewable energy.
The road to reaching those goals presents particularly complex engineering challenges.
“The exciting thing is that we can directly use energy from the sun to drive a chemical reaction, but that means we are asking a single material to do two things, both light harvesting and catalyzing the chemical reaction. No one material is typically good at doing both things. So, you usually have to combine different materials, and how you do that is key to the process,” Muhich said.
In traditional methods of catalyst preparation, a transition metal, like cobalt or platinum, is used to catalyze the reaction, Muhich said. But the problem with that for the combinations of metals he and his colleagues need to make is that the conventional method for their fabrication deposits tens or dozens of atoms in half-sphere-shaped particles.
“Those half-sphere metal objects are good at driving the catalytic reaction but they also undo the light harvesting. So, it essentially turns the light energy into heat, which is of no use, rather than into the chemical reactions you want. You get a little bit of a boost in the catalysis but you lose your light harvesting ability,” he said.
Potential for new source of renewable energy
Christopher Muhich, a Fulton Schools assistant professor of chemical engineering, uses computational chemistry techniques to understand and design materials to advance renewable energy generation and environmental sustainability.
The novelty of the research team’s solution to that problem “is how the catalysts are implemented on the light harvester” that the team is developing, he says.
To achieve both adequate light harvesting and a strong chemical reaction, Yale researchers figured out how to place only a single cobalt atom within a light harvesting sheet of carbon nitride that is just one atom thick. The result is a significant increase in the desired photocatalytic behavior.
The researchers also discovered the photocatalytic process is further enhanced by attaching molecules of organic anthraquinone — a compound that imparts color to plants and is used as a natural dye — around the edges of the carbon nitride sheet.
Muhich and one of his ASU research assistants, chemical engineering doctoral student Srishti Gupta, then provided the mechanistic principles to understand and explain why that system works.
“We used quantum chemistry to model where electrons want to go in this process,” Muhich said. “We used the high-performance computing infrastructure here at ASU to do these gigantic calculations to figure out how electrons distribute themselves in these reactive systems.”
The researchers also see possibilities for using light to drive the reaction of water and oxygen into hydrogen peroxide to generate a source of renewable energy, says Muhich, who teaches in the School for Engineering of Matter, Transport and Energy, one of the six Fulton Schools.
Hydrogen peroxide could be used in a fuel cell to provide energy more efficiently than gasoline. Hydrogen, the conventional fuel used for fuel cells, is a gas that is more difficult to store and transport than a liquid such as hydrogen peroxide. So, hydrogen peroxide could become viable as an alternative fuel for automobiles, Muhich said.
“The heat engines used to convert fuels to electricity in a power plant, or for power in your car have an efficiency of 30% to 35% percent. With hydrogen peroxide in a fuel cell, the conversion of chemical to useful energy could be as high as 80% to 90%, with the added benefit of not having to carry hydrogen gas,” Muhich said.
Scaling up sanitizing technique presents challenges
There are hurdles to realizing the visions for these energy production and water treatment applications. More research is needed to further understand the fundamental chemistry, quantum mechanics and fluid dynamics underlying those photocatalytic processes.
“We need to understand where in the process we are not achieving maximum efficiency, so we can see where and how we need to make improvements,” Muhich said. “We might find, for instance, that there are more efficient materials to use for photocatalysis other than cobalt or anthraquinone.”
The commercialization and infrastructure development needed to bring the benefits of this new water decontamination system to multiple communities will also require major efforts far beyond the laboratory.
“A lot of engineering has to go into designing reactors and photocatalysis operations for introducing light into water and then collecting the hydrogen peroxide that it produces,” Muhich said. “Then there’s also scaling up manufacturing capabilities to an industrial level that can do all of this in a technologically and economically viable way.”
Muhich’s research is bringing other important aspects into the NEWT consortium’s efforts to provide more reliable sources of safe and abundant water, says NEWT’s deputy director, Paul Westerhoff, a Fulton Schools professor of civil, environmental and sustainable engineering.
“Chris brings a unique skillset that enables us to develop machine learning tools to predict interactions of pollutants in water with atomistic differences on the surface of engineered nanoparticles,” Westerhoff said. “NEWT is then using these tools to go from atomistic scale to full-scale engineered treatment processes through other advanced mass transport models.”
Along with “doing exceptional science and being an excellent collaborator,” Westerhoff added, “Chris and his research group passionately embrace and contribute to NEWT’s education outreach and diversity mission.”
More Science and technology

How AI is changing college
Artificial intelligence is the “great equalizer,” in the words of ASU President Michael Crow.It’s compelled industries, including…

The Dreamscape effect
Written by Bret HovellSeventh grader Samuel Granado is a well-spoken and bright student at Villa de Paz Elementary School in…

Research expenditures ranking underscores ASU’s dramatic growth in high-impact science
Arizona State University has surpassed $1 billion in annual research funding for the first time, placing the university among the…