ASU scientists utilize nonconventional techniques to make new materials

Christina Birkel, an inorganic chemist and assistant professor in the School of Molecular Sciences at ASU. Photo by Mary Zhu
Christina Birkel, an inorganic chemist and assistant professor in the School of Molecular Sciences at Arizona State University, is diligently working to create new materials that can be used for renewable energy, catalysts and permanent magnets.
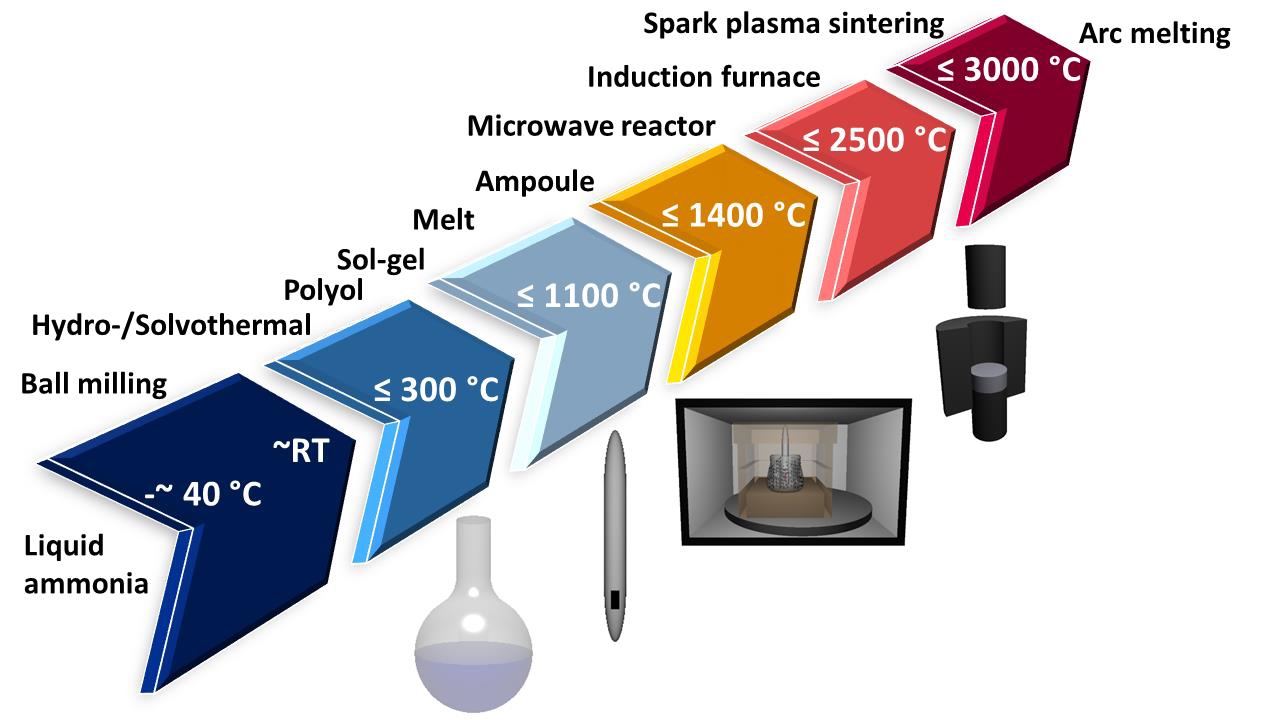
In this age of machine learning and data driven research a plethora of possible materials are being investigated, but synthesizing them in the lab, especially in the solid state, is often anything but straightforward.
"Compared to the highly developed methods for synthesis of molecular systems in solution, the rational synthesis of solid state materials is restricted to a limited number of useful techniques,” explained President’s Professor Ian Gould, interim director of the School of Molecular Sciences, which is part of The College of Liberal Arts and Sciences. “In this paper, Professor Birkel and her students nicely summarize two methods for solid-state synthesis that have already demonstrated potential for the preparation of new high value materials."
“My group is very active in the field of nonconventional synthesis techniques,” Birkel said. “We use microwave heating and spark plasma sintering to synthesize a lot of inorganic — i.e. not derived from living matter- compounds.”
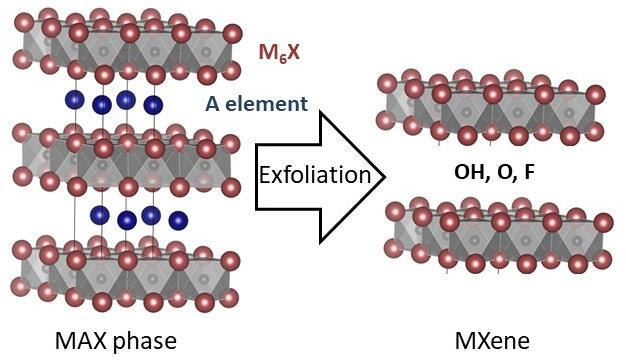
Birkel started out years ago working on oxides and metallic chalcogenides and recently her group has been very active in the field of carbides (so called MAX phases). Solid state microwave heating (using domestic and industrial ovens) is an excellent technique to synthesize carbides. Beautiful layered crystal structures are produced.
The Birkel group performs a lot of electron microscopy, X-ray diffraction and structural analysis on these materials. They are very special in that these materials can be exfoliated, that is, one layer of “A” elements in the structure (usually aluminum) can be etched out very selectively and you can end up with 2D materials (so called “MXenes”). These 2D materials are macroscopic in two dimensions and nanoscopic in the third. These materials have many potential applications including in the field of catalysis.
Birkel’s group is also active in the field of catalysis and is investigating the electrocatalytic properties of these materials, but at the same time they are asking a lot of very fundamental questions about how their synthesis techniques influence the structure and morphology of these compounds.
Where the 2D materials are concerned, it’s important to discover how the surface chemistry evolves during the exfoliation process. They are looking at how they can manipulate the surface chemistry and ultimately how that influences the catalytic properties.
More Science and technology

Making magic happen: Engineering and designing theme parks
The themed entertainment industry is widespread and diverse, encompassing everything from theme parks to aquariums, zoos, water…

AI-equipped feeders allow ASU Online students to study bird behavior remotely
ASU Online students are participating in a research opportunity that's for the birds — literally. Online Bird Buddies is a…

National Humanities Center renews partnership with Lincoln Center for responsible AI research
The National Humanities Center has announced that Arizona State University's Lincoln Center for Applied Ethics is one of four…