Editor's note: This story is being highlighted in ASU Now's year in review. Read more top stories from 2019.
The oldest living organism on Earth is a plant — Methuselah, a bristlecone pine (Pinus longaeva, pictured above) that is more than 5,000 years old. Conversely, animals only live up to a few hundred years. Can we learn something from plants about longevity and stay young forever — or even recapture lost youth?
The Nobel Prize in Physiology or Medicine was awarded in 2009 “for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase.” Telomerase was first isolated from a unicellular organism living in pond scum. As it later turned out, telomerase exists in almost all multicellular organisms, including humans, and plays a crucial role in aging and cancer. Scientists have been scrambling to discover ways to utilize telomerase to make human cells immortal.
For the very first time, a study led by the collaborating groups of Julian Chen in Arizona State University’s School of Molecular Sciences and Dorothy Shippen from Texas A&M University has unraveled the detailed structure and function of the RNA component of telomerase enzyme from land plants. The telomerase RNA components from land plants such as the pine tree show the connection between ciliate (pond scum) and human telomerases and offer new insights into the evolution of telomerase in eukaryotes.
This study, “The conserved structure of plant telomerase RNA provides the missing link for an evolutionary pathway from ciliates to humans,” was just published in the Proceedings of the National Academy of Sciences. The ASU team includes Research Assistant Professor Yang Li and doctoral student Dhenugen Logeswaran.
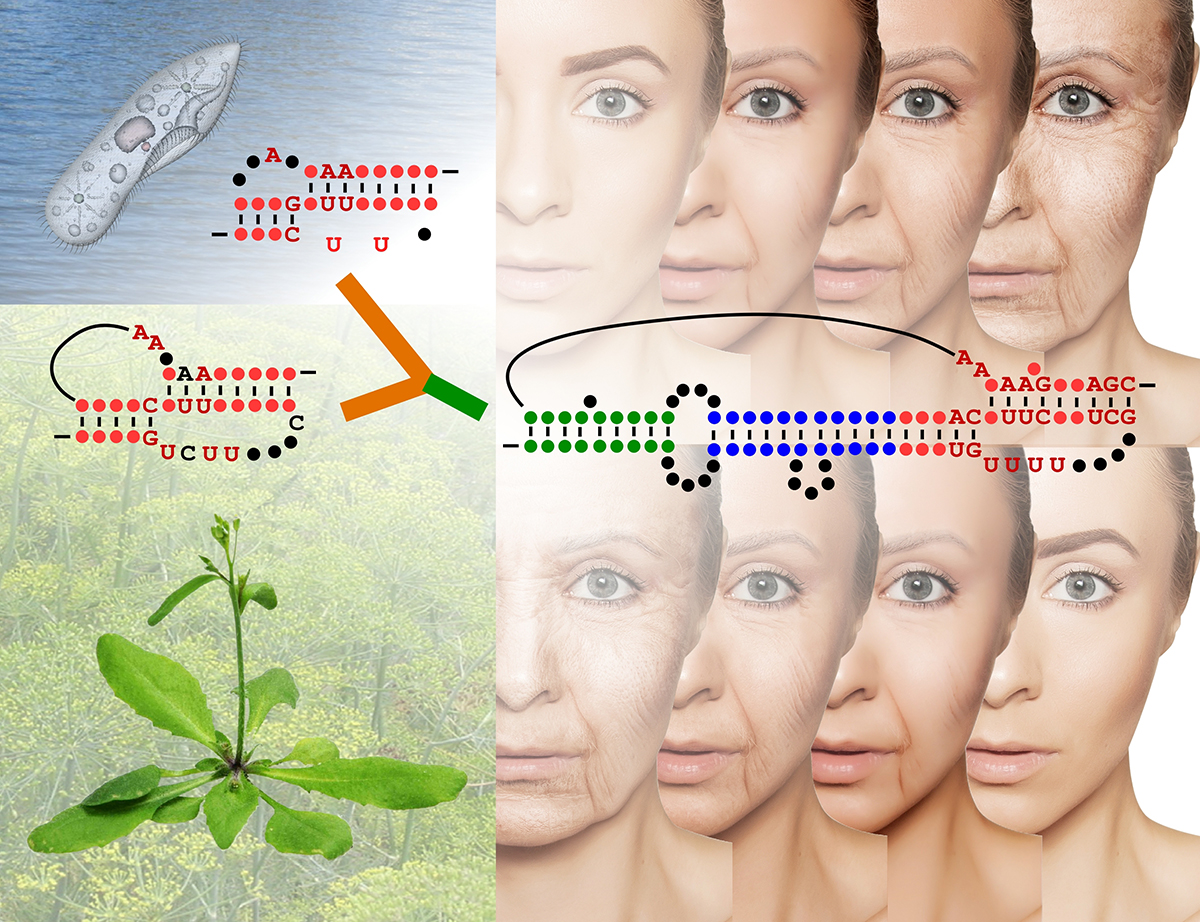
The detailed structure and function of the RNA component of the telomerase enzyme from land plants has just been revealed by the research groups of professors Julian Chen and Dorothy Shippen. Graphic credit: Mary Zhu; image credit: transurfer/stock.adobe.com; ciliate image credit: Wire_man/stock.adobe.com
The fountain of youth at the molecular level
Typical human cells are mortal and cannot forever renew themselves. As demonstrated by Leonard Hayflick a half-century ago, human cells have a limited replicative life span, with older cells reaching this limit sooner than younger cells. This "Hayflick limit" of cellular life span is directly related to the number of unique DNA repeats found at the ends of the genetic material-bearing chromosomes. These DNA repeats are part of the protective capping structures, termed "telomeres," which safeguard the ends of chromosomes from unwanted and unwarranted DNA rearrangements that destabilize the genome.
Each time the cell divides, the telomeric DNA shrinks and will eventually fail to secure the chromosome ends. This continuous reduction of telomere length functions as a "molecular clock" that counts down to the end of cell growth. The diminished ability for cells to grow is strongly associated with the aging process, with the reduced cell population directly contributing to weakness, illness and organ failure.
Counteracting the telomere shrinking process is telomerase, the enzyme that uniquely holds the key to delaying or even reversing the cellular aging process. Telomerase offsets cellular aging by lengthening the telomeres, adding back lost DNA repeats to add time onto the molecular clock countdown, effectively extending the life span of the cell. Telomerase lengthens telomeres by repeatedly synthesizing very short DNA repeats of six nucleotides — the building blocks of DNA — with the sequence "GGTTAG" onto the chromosome ends from a template located within the RNA component of the enzyme itself.
The gradual shrinking of telomeres negatively affects the replicative capacity of human stem cells, the cells that restore damaged tissues and/or replenish aging organs in our bodies. The activity of telomerase in adult stem cells merely slows down the countdown of the molecular clock and does not completely immortalize these cells. Therefore, adult stem cells become exhausted in aged individuals due to telomere length shortening that results in increased healing times and organ tissue degradation from inadequate cell populations.
Identifying telomerase components from land plants
“The study of telomerase has been challenging due to its highly divergent nature across eukaryotic speciesEukaryotes are organisms whose cells have a nucleus enclosed within membranes.,” explained Chen. “The identification of telomerase RNA in land plants is a significant breakthrough in ultimately understanding how this enzyme controls genome stability and cellular immortality in all eukaryotic organisms.”
Professor Julian Chen from the School of Molecular Sciences at ASU.
Telomerase contains two core components for enzymatic function: One is a protein, and the other is an RNA molecule that provides the template for DNA synthesis. RNA and DNA are both nucleic acids that can store genetic sequence information. The RNA component of telomerase is extremely divergent in sequence and difficult to find across eukaryotic organisms. In fact, an incorrect RNA molecule was reported previously as the plant telomerase RNA nearly a decade ago, and it has taken until today to discover the correct one.
The RNA components of telomerases from both free-living unicellular pond scum and humans show marked differences. Plant telomerase RNA, interestingly, shares features of telomerase RNAs from both pond scum and from humans; i.e., a “missing link.”
Tapping the full potential of telomerase
Understanding the regulation and limitation of the telomerase enzyme holds the promise of reversing telomere shortening and cellular aging with the potential to extend human life span and improve the health and wellness of elderly individuals.
Human diseases that include dyskeratosis congenita, aplastic anemia and idiopathic pulmonary fibrosis have been genetically linked to mutations that negatively affect telomerase activity and/or accelerate the loss of telomere length. This accelerated telomere shortening closely resembles premature aging with increased organ deterioration and a shortened patient life span caused by critically insufficient cell populations. Increasing telomerase activity is seemingly the most promising means of treating these diseases.
While increased telomerase activity could bring youth to aging cells and cure premature aging-like diseases, too much of a good thing can be damaging for the individual. Just as youthful stem cells use telomerase to offset telomere length loss, cancer cells employ telomerase to maintain their aberrant and destructive growth. Augmenting and regulating telomerase function will have to be performed with precision, walking a narrow line between cell rejuvenation and a heightened risk for cancer development.
Distinct from human stem cells, somatic cells constitute the vast majority of the cells in the human body and lack telomerase activity. The telomerase deficiency of human somatic cells reduces the risk of cancer development, as telomerase fuels uncontrolled cancer cell growth. Therefore, drugs that increase telomerase activity indiscriminately in all cell types are not desired. Small molecule drugs can be screened or designed to increase telomerase activity exclusively within stem cells for disease treatment as well as antiaging therapies without increasing the risk of cancer.
The study of plant telomerase may offer new directions on how to modulate or engineer human telomerase for innovations in developing antiaging and anticancer therapeutics.
This research was supported by National Institutes of Health Grants R01GM094450 (to J.J.-L.C.) and R01GM065383 (to D.E.S), and National Science Foundation Grants MCB1616078 (to J.J.-L.C.) and MCB151787 (to D.E.S).
Top photo: Methuselah, a bristlecone pine (Pinus longaeva) that is more than 5,000 years old. Photo by shoenberg3/stock.adobe.com
More Science and technology
From wannabe high school math teacher to Regents Professor
It’s a bit difficult to describe the work Stephanie Forrest does as director of Arizona State University’s Biodesign Center for Biocomputation, Security and Society.Her ASU biography lays it out this…
Regents Professor recognized as pioneer in educational technology
Reading can transport people around the world — and back and forth in time.That’s what Danielle McNamara has helped tens of thousands of students do.McNamara is the executive director and…

Professor’s expertise in technology transfer leads to top faculty honor
Universities spend billions of dollars on research, and the process of transforming that work into goods and services for the public is more important than ever.ASU Professor Donald Siegel is an…

