ASU study unveils role of microglial cells, viral linkage in Alzheimer’s, Parkinson’s disease

Schematic of laser capture microdissection on hippocampal tissue sections (A). Hippocampal sections were exposed to an antibody to LN3 (B), and ~600 LN3 positive microglia cells were captured (C). Individual microglial cells were cut and dropped into an inverted microcentrifuge cap (D) and processed for RNA sequencing.
In their attempts to untangle the mystery of Alzheimer’s disease, researchers have traditionally focused on damage to the basic building blocks of thought — the neurons. Numbering close to a hundred billion, neurons form a communications network of unmatched complexity, helping to oversee essential physical functions and acting as repositories of our identity, emotions and memory.
More recently however, interest in the non-neuronal cells inhabiting the central nervous system has greatly expanded. Collectively known as glia, these diverse cell types perform a range of tasks and play vital roles in the maintenance of neural health and the progression of brain disease.
In a new study, researchers at the ASU-Banner Neurodegenerative Disease Research Center investigate a subclass of glial cells known as microglia. Neglected, yet crucial players in protecting the healthy brain from injury and infection, they may also contribute to neurodegenerative diseases and other pathologies.
Samples of microglia from the brains of both Alzheimer’s and Parkinson’s disease patients showed alterations in the gene expression of microglial cells, compared with normal samples. The altered cell expression levels were found to be specific to different locations in the brain.
Further, patterns of microglia expression in Alzheimer’s samples pointed to two prominent neural pathways, the first related to neuronal repair and the second to viral processing.
Intriguingly, microglia samples from brains testing positive for Alzheimer’s disease appeared to display immune activity associated with the hepatitis B virus, suggesting that carriers of the virus may be at heightened risk for developing Alzheimer’s.
“Our unexpected finding of neurodegenerative risk in hepatitis B carriers may have a profound effect on those countries where hepatitis B is epidemic,” said Diego Mastroeni, a lead author of the new study.
Diego Mastroeni is a researcher in the ASU-Banner Neurodegenerative Disease Research Center.
Mind matters
The study marks the first research effort focusing on the behavior of microglia drawn from differing brain regions and disease types and offers a new wrinkle in the story of one of the most devastating and intractable diseases.
The researchers were able to examine microglia from normal, Alzheimer’s and Parkinson’s brains once they had been isolated from other cells types, using a leading-edge technology known as laser capture microdissection.
“We characterize microglia from different brain regions and in different pathologies in humans and demonstrate that microglia exhibit different gene expression patterns depending on the brain region and disease,” Mastroeni said. “This corroborates and expands our view that microglia are not homogenous but highly heterogeneous populations of cells.”
The research findings recently appeared in the journal Neurobiology of Aging.
Identity theft
Alzheimer’s disease, the leading cause of dementia, produces catastrophic and comprehensive changes to the brain and human behavior. The disease is typically fatal within three to nine years of diagnosis. But today, researchers know that the illness begins to subtly, imperceptibly seize control of the brain decades before the arrival of telltale symptoms: increasing memory loss, confusion, agitation and mood swings, loss of communication skills and motor system deficit.
Alzheimer’s afflicts some 44 million worldwide. In the U.S., 5.5 million are stricken with Alzheimer’s and the disease is in steep ascent, due to an aging population. The trends are chilling, with projections of up to 16 million cases in the U.S. alone by 2050, exacting a devastating human and economic toll, unless major advances can halt the illness in its tracks or a preventive strategy can be found.
Unfortunately, the multiple transformations leading to Alzheimer’s are still largely in the shadows, and despite massive investments of resources, the disease remains the only leading killer without any means of prevention, treatment or cure.
Paul Coleman is a researcher in the ASU-Banner Neurodegenerative Disease Research Center.
The prevailing hypothesis focuses on two common features often found in brains afflicted with Alzheimer’s. The first is concentrations of a sticky protein known as amyloid beta, which over time accumulates to form plaque-like concentrations in the brain tissue, ultimately leading to widespread neuronal death. The other diagnostic marker is found within neuronal cell bodies themselves and takes the form of tangle-like structures of another protein known as tau.
Intensive study of these common features of Alzheimer’s have done much to advance the field, though a raft of amyloid- and tau-targeting drugs have failed in clinical trials and thus far appear to offer no measurable benefit. It now seems likely that plaques and tangles are late arrivals in the cascade of events leading to dementia.
New approaches are desperately needed in the fight against Alzheimer’s, particularly those focused on the earliest precursors of the disease.
Double-edged cells
Glia take their name from the Greek word meaning “glue.” They are a family of cells of differing shapes, sizes and functions in the brain. While it is true that their role in part is to surround the brain’s forest of neurons and hold them in place, research since the turn of the 20th century has pointed to the increasing importance of these cells in many other critical activities.
This is especially true of the microglia, which were meticulously observed between 1919 and 1921 by Pío del Río Hortega, a student of the great Spanish neuroanatomist Santiago Ramón y Cajal. Thus began the serious study of microglia and the modern conception of their nature.
As their name suggests, microglia tend to be smaller than astrocytes, oligodendrites and other members of the glial family. They play a commanding role in the brain’s immune system, ceaselessly patrolling the nervous system for signs of infection or trauma and clearing away damage and debris. Their activity is often compared with macrophages — a type of white blood cell central to the body’s innate immune system. Like macrophages, microglia are known to engulf and ingest foreign substances and microbes, cellular debris and aberrant cells like cancer, through a process known as phagocytosis.
The life of microglial cells begins in blood islands of the yolk sac during early fetal development. Early in the brain’s formation, microglia are involved in dendritic pruning and modeling activity that will help fashion the adult brain. From their domain of origin, microglia migrate to colonize the entire brain and spinal cord. They are most abundant in the hippocampus, olfactory telencephalon, basal ganglia and substantia nigra.
Despite their essential role as the brain’s homeland security system, microglia’s defensive arsenal can go awry, causing serious harm to the brain they are designed to nurture and protect.
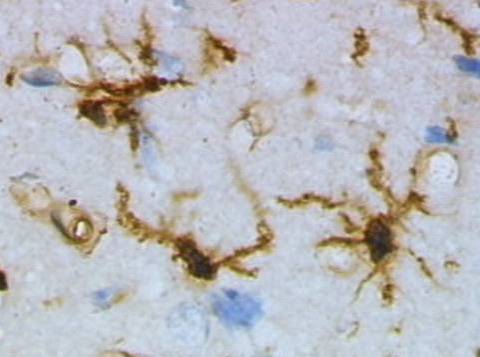
Microglia seen in their resting state from rat cortex. Microglia act as the brain's immune system, migrating to areas of trauma and disease, altering their shape and releasing a broad range of chemicals, including cytotoxic factors to destroy invaders.
Shape shifters
In defending the most important organ in the body, microglia rely on a formidable array of tactics researchers are just beginning to understand. Microglia, which represent about 10 percent of the brain’s complement of glial cells, have a protean form, allowing them to change their shape as conditions dictate.
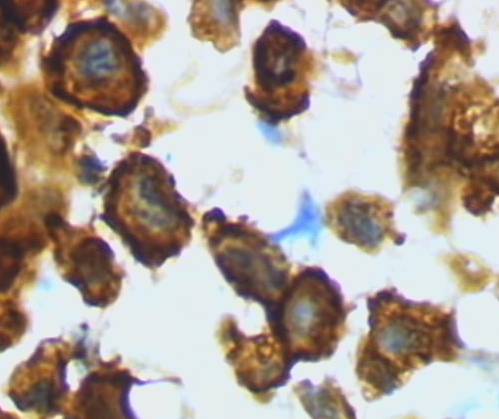
Three states of microglia are recognized, resting, active, and amoeboid. In their resting condition, microglia display small cell bodies and long branching tendrils or processes that delicately sense their intercellular fluid environment, alert to anything amiss. When they encounter trauma or foreign infection, they retract their processes and assume a blob-like, active form as they search out inflammation associated with injury or infection.
When microglia encounter foreign cells like bacteria, they unleash a kind of chemical warfare, producing a disparate range of cytotoxic substances including cytokines, chemokines and reactive oxygen species capable of destroying the invader. Dead cells and debris are then cleared away by microglia that swallow and digest them through phagocytosis.
Microglia then export pieces of the internalized debris to their cell surfaces, allowing other cells of the immune system like lymphocytes to read them and mount further defensive measures. Immune cells further increase inflammation, thereby activating other microglia and luring them to the defense. Should these mechanisms get out of balance however, microglia can turn hostile to the brain they are charged with protecting, and in the case of certain diseases, execute neuronal destruction.
Microglia in activated form from rat cortex, responding to injury or infection.
Targeting microglia
In the new study, single-cell laser capture methods isolated microglia from normal elderly patients as well as from Parkinson’s and Alzheimer’s brains. The microglia were drawn from two distinct brain regions known to be affected by Alzheimer’s and Parkinson’s, the hippocampus and substantia nigra.
Conventional methods for analyzing cells from brain tissue rely on preparing homogenates, which involves grinding tissue composed of many cell types into a creamy consistency before evaluating gene expression. Despite the usefulness of the method in many studies, it can obscure the subtleties of gene expression seen in individual cell types and microglia-specific information is often lost, as these cells are outnumbered in homogenates by other cell types.
Indeed, laser capture enabled the researchers to pick out the range of subtle alterations in microglia, noting important expression changes in genes associated with neuronal repair as well as the unexpected finding that pathways of viral processing were activated in AD. Further, reactivity to the hepatitis B virus was shown to be proportional to the degree of Alzheimer’s severity.
The findings significantly deepen the understanding of microglia, as previous research based on homogenates focused almost exclusively on inflammation-related transcripts. The authors note that despite the importance of inflammation triggered by microglial activity, inflammation-related gene transcripts represent a small fraction of the roughly 20,000 protein coding transcripts attributed to microglial cells.
“Descriptive studies like these, using optimal postmortem human brain material, coupled with imaginative analyses of the data resulting from these ‘experiments of nature’ can lead to major understanding of how glial cells respond in disease and, consequently, the creation of vastly improved model systems,” Mastroeni said.
More Science and technology

Teaching construction realities with virtual environments
Visiting a construction site is a valuable learning opportunity for students who want to one day work in the industry.…
ASU, Mexico partner to build next generation of chipmakers, drive semiconductor innovation
Thousands of college students in Mexico will soon have the opportunity to enroll in Arizona State University’s new, free online…

ASU, St. Mary’s Food Bank partner to tackle food insecurity in Arizona
Arizona State University and St. Mary’s Food Bank (SMFB) have joined forces to create an interactive data dashboard that tracks…