Experimental drug shown to protect energy powerhouses in brain, a promising step in fight against Alzheimer’s
In a new study, a team of Arizona State University researchers have examined the effects of Alzheimer’s disease to rob brain cells of their primary energy source — decades before symptoms first appear.
And in a promising finding, the researchers have shown for the first time that human neuronal cells can be protected from OAβ-induced destruction of brain cells when they are pretreated with a custom-designed experimental compound.
The new research reveals that a highly toxic form of a protein implicated in Alzheimer’s — a version of the beta amyloid protein known as oligomeric a-beta (OAβ) — disrupts the normal functioning of mitochondria, structures that are the powerhouses of every cell.
Diego Mastroeni
“Mitochondria are the major source of energy in brain cells, and deficiencies in energy metabolism have been shown to be one of the earliest events in Alzheimer's disease pathobiology,” said Diego Mastroeni, a scientist at the ASU-Banner Neurodegenerative Disease Research Center and lead author of the new study.
The compound, which was designed by study co-author and ASU Biodesign Institute collaborator Sidney Hecht, acted to protect cells from the degradation normally caused to mitochondrial function from OAβ, offering renewed hope for effective treatment.
The research findings appear in "Oligomeric Amyloid Beta Preferentially Targets Neuronal and Not Glial Mitochondrial Encoded mRNAs," made available online by Alzheimer's & Dementia: The Journal of the Alzheimer's Association.
Mind out of step
Alzheimer's disease affects millions worldwide. To date, clinical efforts to find a cure or adequate treatment have met with dispiriting failure.
The disease is now on an ominous course of expansion, due in part to an aging population, and is poised to become a global health emergency. The enigmatic ailment — first described more than 100 years ago — remains the leading killer without any effective treatment, prevention or cure.
The prevailing theory points to accumulations of a sticky protein substance known as amyloid beta in the brain, initiating the chain of events leading to development of Alzheimer's disease.
But the continued failure of clinical trials that target amyloid beta to try and alter the course of Alzheimer’s disease has forced scientists to go back to the drawing board.
Power outage
Alzheimer's appears to selectively target neurons for destruction, and those found in the hippocampus — an area of the brain associated with memory — appear to be particularly vulnerable.
One important scientific clue is the way our brains hog energy.
Although the human brain represents only 2 percent of the body's weight, it accounts for a full 20 percent of the body's total oxygen consumption.
The brain's forest of neurons require a prodigious amount of energy for their electrochemical cross talk. And the brain's intense thirst for energy is continuous, and even brief periods of sugar or oxygen deprivation can result in neuronal death.
The ASU research team looked at the vulnerability of the energy sources in every cell, found in structures called mitochondria, which are currently under intense investigation for their early role in Alzheimer’s pathology.
New clues
In the new study, cells known as pyramidal neurons, isolated from the hippocampus of patients who died of Alzheimer's, were shown to have a marked reduction in the expression of a suite of mitochondrial genes, pointing to their degradation by OAβ.
Furthermore, the reduction of mitochondrial gene expression was also seen when cells belonging to a human neuroblastoma cell line were exposed to OAβ.
In complimentary experiments, the group used human neuroblastoma cells exposed to OAβ. Compared with AD neurons, the neuroblastoma cells showed a similar reduction in the expression of specific mitochondrial-encoded genes — strong circumstantial support for OAβ’s injurious effects on mitochondria.
The overlapping effects on gene expression clearly seen in both the AD and OAβ-treated neuroblastoma cells point to OAβ’s selective assault on the nervous system’s energy supply, opening the door for targeted therapy.
Next, the human brain cells were pre-treated in the lab with an analog of CoQ10, a structural compound capable of protecting and boosting mitochondrial energy production. When this was done prior to their exposure to OAβ, the drug acted to protect cells.
This offers renewed hope to develop an effective Alzheimer’s treatment.
A new avenue
While the researchers are optimistic, the progression of Alzheimer’s disease will likely not have a single root cause. The authors stress that not all types of nervous system cells are implicated in the mitochondrial dysfunction brought on by exposure to OAβ.
Important support and repair brain cells, including hippocampal astrocyte and microglia cells taken from the same Alzheimer’s-afflicted brains, did not display reduced mitochondrial function.
But the study further establishes mitochondrial deficit caused by exposure to OAβ as a highly promising avenue for further research in the ongoing battle against this devastating illness.
“This study reinforces the toxicity of oligomeric amyloid beta on neuronal mitochondria and stresses the importance for protective compounds to protect the mitochondria from oligomeric amyloid beta toxicity," said Mastroeni, who is also an assistant research professor in the School of Life Sciences.
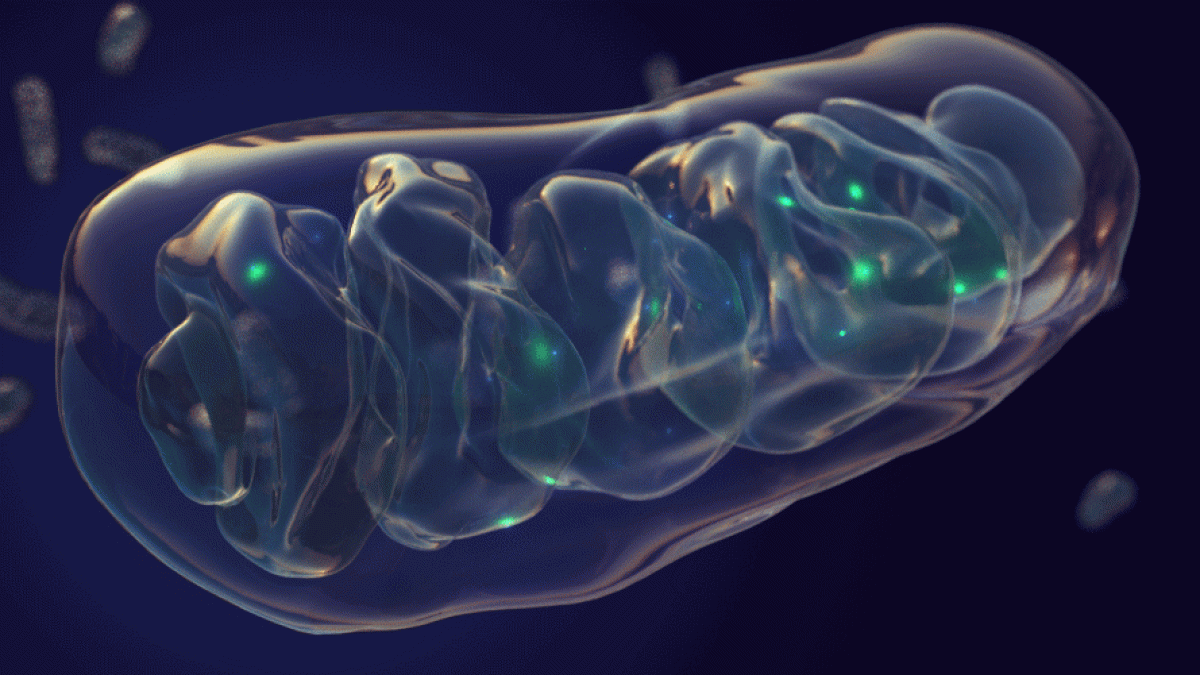
Top image: Mitochondria are the energy factories of the brain. New research suggests they may play an intimate role in Alzheimer's disease. Graphic by Jason Drees/ASU
More Science and technology

Applied Materials invests in ASU to advance technology for a brighter future
For nearly 60 years, global giant Applied Materials has been hard at work engineering technology that continues to change how microchips are made.Their products power everything from flat-panel…

Meet ASU engineering students who are improving health care, computing and more
Furthering knowledge of water resource management, increasing the efficiency of manufacturing point-of-care health diagnostic tools and exploring new uses for emerging computer memory are just some…

Turning up the light: Plants, semiconductors and fuel production
What can plants and semiconductors teach us about fuel production?ASU's Gary Moore hopes to find out.With the aim of learning how to create viable alternatives to fossil-based fuels, Moore — an…


