What if one day, we could teach our bodies to self-heal like a lizard’s tail, and make severe injury or disease no more threatening than a paper cut?
Or heal tissues by coaxing cells to multiply, repair or replace damaged regions in loved ones whose lives have been ravaged by stroke, Alzheimer’s or Parkinson’s disease?
Such is the vision, promise and excitement in the burgeoning field of regenerative medicine, now a major ASU initiative to boost 21st-century medical research discoveries.
ASU Biodesign Institute researcher Nick Stephanopoulos is one of several rising stars in regenerative medicine. In 2015, Stephanopoulos, along with Alex Green and Jeremy Mills, were recruited to the Biodesign Institute’s Center for Molecular Design and Biomimetics (CMDB), directed by Hao Yan, a world-recognized leader in nanotechnology.
“One of the things that that attracted me most to the ASU and the Biodesign CMDB was Hao’s vision to build a group of researchers that use biological molecules and design principles to make new materials that can mimic, and one day surpass, the most complex functions of biology,” Stephanopoulos said, also an assistant professor in the School of Life Sciences.
“I have always been fascinated by using biological building blocks like proteins, peptides and DNA to construct self-assembled structures, devices and materials, and the interdisciplinary and highly collaborative team in the CMDB is the ideal place to put this vision into practice.”
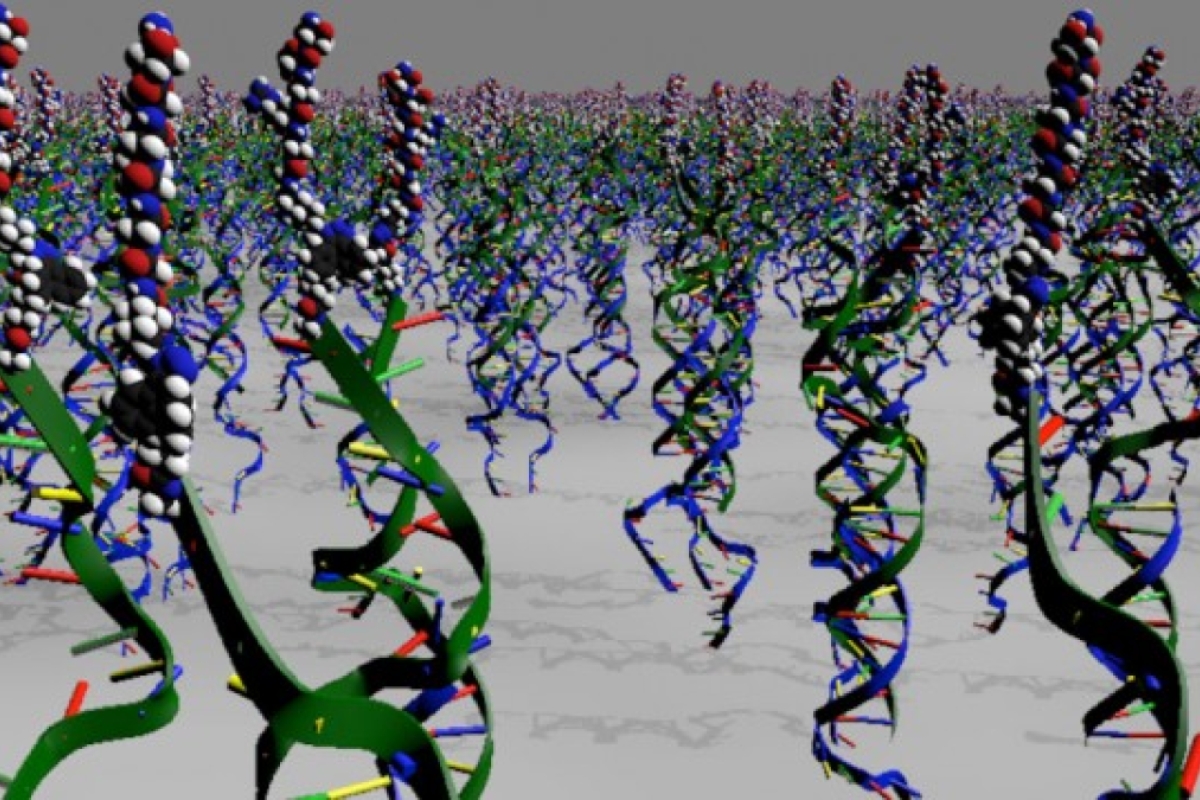
Yan’s research center uses DNA and other basic building blocks to build their nanotechnology structures — only at a scale 1,000 times smaller than the width of a human hair.
They’ve already used nanotechnology to build containers to specially deliver drugs to tissues, build robots to navigate a maze or nanowires for electronics.
To build a manufacturing industry at that tiny scale, their bricks and mortar use a colorful assortment of molecular Legos. Just combine the ingredients, and these building blocks can self-assemble in a seemingly infinite number of ways only limited by the laws of chemistry and physics — and the creative imaginations of these budding nano-architects.
Learning from nature
“The goal of the Center for Molecular Design and Biomimetics is to use nature’s design rules as an inspiration in advancing biomedical, energy and electronics innovation through self-assembling molecules to create intelligent materials for better component control and for synthesis into higher-order systems,” said Yan, who also holds the Milton Glick Chair in Chemistry and Biochemistry.
Prior to joining ASU, Stephanopoulos trained with experts in biological nanomaterials, obtaining his doctorate with the University of California Berkeley’s Matthew Francis, and completed postdoctoral studies with Samuel Stupp at Northwestern University. At Northwestern, he was part of a team that developed a new category of quilt-like, self-assembling peptide and peptide-DNA biomaterials for regenerative medicine, with an emphasis in neural tissue engineering.
“We’ve learned from nature many of the rules behind materials that can self-assemble. Some of the most elegant complex and adaptable examples of self-assembly are found in biological systems,” Stephanopoulos said.
Because they are built from the ground-up using molecules found in nature, these materials are also biocompatible and biodegradable, opening up brand-new vistas for regenerative medicine.
Stephanopoulos’ tool kit includes using proteins, peptides, lipids and nucleic acids like DNA that have a rich biological lexicon of self-assembly.
“DNA possesses great potential for the construction of self-assembled biomaterials due to its highly programmable nature; any two strands of DNA can be coaxed to assemble to make nanoscale constructs and devices with exquisite precision and complexity,” Stephanopoulos said.
Proof all in the design
During his time at Northwestern, Stephanopoulos worked on a number of projects and developed proof-of-concept technologies for spinal cord injury, bone regeneration and nanomaterials to guide stem cell differentiation.
Now, more recently, in a new study in Nature Communications, Stephanopoulos and his colleague Ronit Freeman in the Stupp laboratory successfully demonstrated the ability to dynamically control the environment around stem cells, to guide their behavior in new and powerful ways.
In the new technology, materials are first chemically decorated with different strands of DNA, each with a unique code for a different signal to cells.
To activate signals within the cells, soluble molecules containing complementary DNA strands are coupled to short protein fragments, called peptides, and added to the material to create DNA double helices displaying the signal.
By adding a few drops of the DNA-peptide mixture, the material effectively gives a green light to stem cells to reproduce and generate more cells. In order to dynamically tune the signal presentation, the surface is exposed to a soluble single-stranded DNA molecule designed to “grab” the signal-containing strand of the duplex and form a new DNA double helix, displacing the old signal from the surface.
This new duplex can then be washed away, turning the signal “off.” To turn the signal back on, all that is needed is to now introduce a new copy of single-stranded DNA bearing a signal that will reattach to the material’s surface.
One of the findings of this work is the possibility of using the synthetic material to signal neural stem cells to proliferate, then at a specific time selected by the scientist, trigger their differentiation into neurons for a while, before returning the stem cells to a proliferative state on demand.
One potential use of the new technology to manipulate cells could help cure a patient with neurodegenerative conditions like Parkinson’s disease.
The patient’s own skin cells could be converted to stem cells using existing techniques. The new technology could help expand the newly converted stem cells back in the lab — and then direct their growth into specific dopamine-producing neurons before transplantation back to the patient.
“People would love to have cell therapies that utilize stem cells derived from their own bodies to regenerate tissue,” Stupp said. “In principle, this will eventually be possible, but one needs procedures that are effective at expanding and differentiating cells in order to do so. Our technology does that.”
In the future, it might be possible to perform this process entirely within the body. The stem cells would be implanted in the clinic, encapsulated in the type of material described in the new work, and injected into a particular spot. Then the soluble peptide-DNA molecules would be given to the patient to bind to the material and manipulate the proliferation and differentiation of transplanted cells.
Scaling the barriers
One of the future challenges in this area will be to develop materials that can respond better to external stimuli and reconfigure their physical or chemical properties accordingly.
“Biological systems are complex, and treating injury or disease will in many cases necessitate a material that can mimic the complex spatiotemporal dynamics of the tissues they are used to treat,” Stephanopoulos said.
It is likely that hybrid systems that combine multiple chemical elements will be necessary; some components may provide structure, others biological signaling and yet others a switchable element to imbue dynamic ability to the material.
A second challenge, and opportunity, for regenerative medicine lies in creating nanostructures that can organize material across multiple length scales. Biological systems themselves are hierarchically organized: from molecules to cells to tissues, and up to entire organisms.
Consider that for all of us, life starts simple, with just a single cell. By the time we reach adulthood, every adult human body is its own universe of cells, with recent estimates of 37 trillion or so. The human brain alone has 100 billion cells or about the same number of cells as stars in the Milky Way galaxy.
But over the course of a life, or by disease, whole constellations of cells are lost due to the ravages of time or the genetic blueprints going awry.
Collaborative DNA
To overcome these obstacles, much more research funding and recruitment of additional talent to ASU will be needed to build the necessary regenerative medicine workforce.
Last year, Stephanopoulos’ research received a boost with funding from the U.S. Air Force’s Young Investigator Research Program (YIP).
“The Air Force Office of Scientific Research YIP award will facilitate Nick’s research agenda in this direction, and is a significant recognition of his creativity and track record at the early stage of his careers,” Yan said.
They’ll need this and more to meet the ultimate challenge in the development of self-assembled biomaterials and translation to clinical applications.
Buoyed by the funding, during the next research steps, Stephanopoulos wants to further expand horizons with collaborations from other ASU colleagues to take his research team’s efforts one step closer to the clinic.
“ASU and the Biodesign Institute also offer world-class researchers in engineering, physics and biology for collaborations, not to mention close ties with the Mayo Clinic or a number of Phoenix-area institutes so we can translate our materials to medically relevant applications,” Stephanopoulos said.
There is growing recognition that regenerative medicine in the Valley could be a win-win for the area, in delivering new cures to patients and building, person by person, a brand-new medicinal manufacturing industry.
Stephanopoulos’ recent research was carried out at Stupp’s Northwestern’s Simpson Querrey Institute for BioNanotechnology. The National Institute of Dental and Craniofacial Research of the National Institutes of Health (grant 5R01DE015920) provided funding for biological experiments, and the U.S. Department of Energy, Office of Science, Basic Energy Sciences provided funding for the development of the new materials (grants DE-FG01-00ER45810 and DE-SC0000989 supporting an Energy Frontiers Research Center on Bio-Inspired Energy Science (CBES)).
The paper is titled “Instructing cells with programmable peptide DNA hybrids.” Samuel I. Stupp is the senior author of the paper, and post-doctoral fellows Ronit Freeman and Nicholas Stephanopoulos are primary authors.
More Science and technology

ASU technical innovation enables more reliable and less expensive electricity
Growing demand for electricity is pushing the energy sector to innovate faster and deploy more resources to keep the lights on and costs low. Clean energy is being pursued with greater fervor,…

What do a spacecraft, a skeleton and an asteroid have in common? This ASU professor
NASA’s Lucy spacecraft will probe an asteroid as it flys by it on Sunday — one with a connection to the mission name.The asteroid is named Donaldjohanson, after Donald Johanson, who founded Arizona…

Hack like you 'meme' it
What do pepperoni pizza, cat memes and an online dojo have in common?It turns out, these are all essential elements of a great cybersecurity hacking competition.And experts at Arizona State…