The tidal wave approaches. In the coming decades, Alzheimer's disease is projected to exact a devastating economic and emotional toll on society, with patient numbers in the U.S. alone expected to reach 13.5 million by midcentury at a projected cost of more than a trillion dollars.
Salvatore Oddo (left), a researcher at Biodesign Neurodegenerative Disease Research CenterThe Biodesign Neurodegenerative Disease Research Center is a partnership between ASU and Phoenix-based Banner Health. and Arizona State University associate professor, has been investigating the underpinnings of this devastating illness.
In new research appearing in the journal Molecular Psychiatry, Oddo and his colleagues examine a critical protein associated with telltale symptoms of Alzheimer's. They have shown that the protein p62 is associated with the accumulation of plaques formed in the brain from deposits of another crucial protein known as amyloid beta.
The study holds clues for reversing the effects of damaging plaques in the brain by demonstrating for the first time that this protein regulates the degradation, or turnover, of amyloid beta in living systems.
"These exciting finding suggest that compounds aimed at increasing p62 may have beneficial effects for Alzheimer's disease," Oddo said.
The multi-purpose protein p62 has already been associated with the other classic neurological symptoms of Alzheimer's. Known as neurofibrillary tangles, these twisted strands of tau protein form inside dying cells, destroying vital pathways for nutrients.
Unremitting destruction
Alzheimer's disease causes nerve cell death and tissue loss throughout the brain. During the course of the disease, the brain shrinks dramatically, affecting nearly all its functions. Shrinkage of the brain is acute in the cortex, damaging areas critical for thinking, planning and remembering. Atrophy is particularly severe in the hippocampus, an area of the cortex that plays a crucial role in formation of new memories. By contrast, the fluid-filled spaces in the brain known as ventricles grow larger.Alzheimer's is on a rapid and devastating assent, due in part to an aging U.S. population and greater average lifespans. One in three seniors dies with Alzheimer's or a related form of dementia. New cases of the ailment occur every 66 seconds, with the pace accelerating to 1 every 33 seconds by 2050. Environmental factors ranging from diet and exercise to long-term health conditions, including high blood pressure and diabetes, may also play a role.
In addition to the catastrophic effects of Alzheimer's on the lives of patients, the disease places overwhelming stress on immediate caregivers, often leading to depression and, in some cases, economic bankruptcy.
In 2015, 15.9 million family members and friends of those suffering from Alzheimer's and other forms of dementia provided a staggering 18.1 billion hours of unpaid care with an economic value of $221.3 billion.
While commonly described as a disease of old age, early-onset forms can strike in midlife. Further, an increasing body of research suggests the ravaging of the brain caused by the disease actually begins decades before the onset of clinical symptoms.
A clue emerges
The protein p62 is known to perform an array of vital functions in cells. Of particular interest is p62's role in the aggregation and degradation of a pair of proteins long recognized as hallmarks of Alzheimer's disease — amyloid beta and tau.
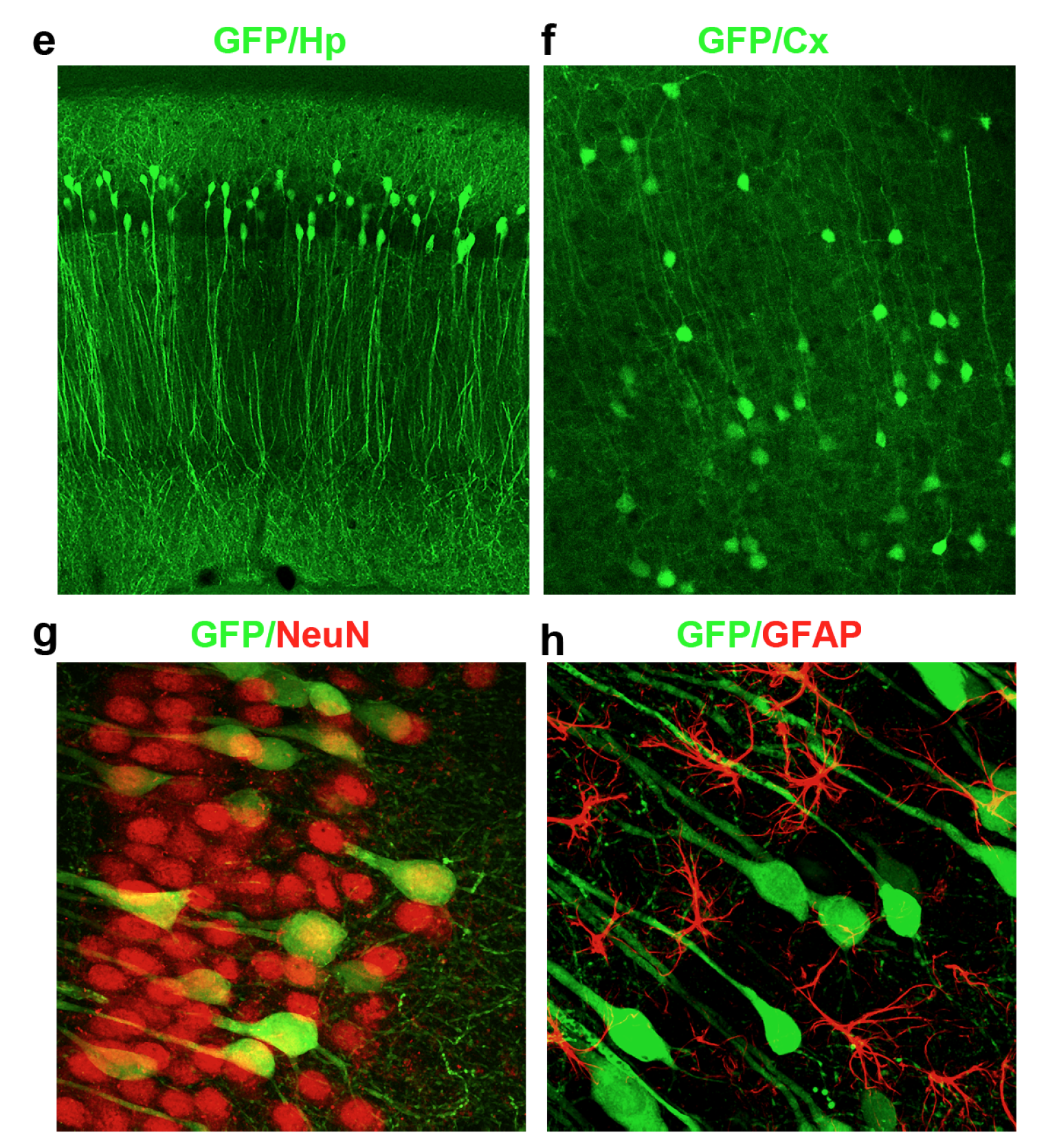
A virus loaded with the protective protein p62 was infected into mice. The green (e, f) and red stains (g, h) show that the virus can infect neurons in the hippocampus and cortex, important regions of the brain implicated in Alzheimer’s disease.
The authors demonstrate, for the first time, that a modified strain of mice generated to display human-like symptoms of Alzheimer's show significant cognitive improvements, including a reversal of spatial memory deficit, when the brain's expression of p62 is restored.
The study further shows that the improvement is associated with reduced levels of amyloid beta and associated plaques in the brain. Finally, the new research describes the mechanism by which p62 activity improves Alzheimer's disease symptoms in mice — by a process known as autophagy. The term refers to the degradation or disassembly of unnecessary or dysfunctional components of cells, a form of biological recycling essential for cellular health.
Reduce, reuse, recycle
Maintenance of human health requires intensive and ongoing cleanup operations. Proteins essential to life processes must be degraded after fulfilling their assigned tasks, which range from providing structural support to cells and tissues to protecting the body from pathogenic foreign invaders. If the body's housekeeping operations are stalled or inefficient, the results can be disastrous.
Previous research has revealed how imbalances between protein production and degradation can lead to accumulations of protein products associated with several deadly neurodegenerative disorders, including frontotemporal lobar degeneration, amyotrophic lateral sclerosis and Alzheimer's disease.
During the process of autophagy, unwanted constituents of cells are isolated and walled off in specialized double-membraned compartments known as autophagosomes. The packaged protein detritus then fuses with lysosomes, organelles in the cell's cytoplasm whose digestive enzymes break down protein components. Recycling is completed when constituent amino acids from degraded proteins become the raw material for new proteins.
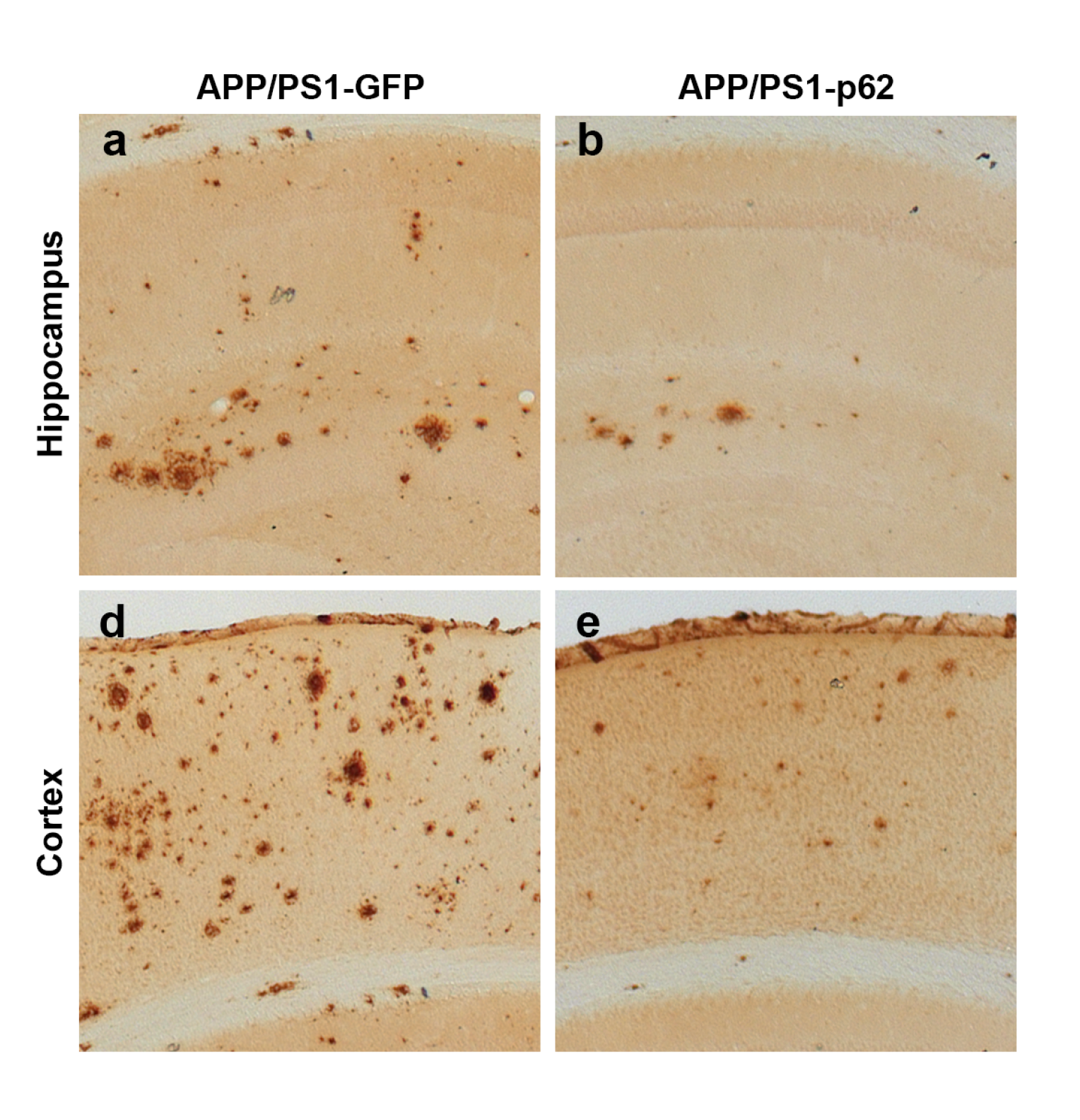
Microphotographs of the brain show that the presence of p62 in the brain can dramatically reduce the amount of amyloid beta protein (Aβ42) both in the hippocampus (a,b) and cortex (d, e). Amyloid protein is associated with the development of harmful Alzheimer’s disease plaques.
The study observed the behavior of mice genetically bred to lack the p62 protein, resulting in age-dependent cognitive deficits. Earlier observations pointed to the underlying role of p62 in regulating the accumulation of tau protein in the brain. As the authors note, p62 binds strongly to these tau tangles in Alzheimer's, most likely in order to mark them for degradation.
Memory and cognition were assessed through a simple test in which the mice had to mentally recall the locations of submerged platforms in a circular water maze. P62 was introduced to the transgenic mice through a virus specifically designed to carry it into the mouse brain.
Mice received four training trials per day in the water maze for five consecutive days. While all the mice improved over the five-day trials, those expressing p62 performed markedly better, displaying restoration of cognitive skills. The mode of action of p62 appears to be the induction of autophagy and the delivery of amyloid beta to lysosomes for processing and breakdown, thereby reversing the buildup of amyloid beta associated with cognitive impairment.
These data indicate that p62 leads to the induction of autophagy and facilitates the delivery of protein byproducts to lysosomes. Loss of p62 function in Alzheimer's disease, perhaps due to oxidative damage of its promoter, is linked with the accumulation of both plaques and tangles.
The research results open the possibility of restoring neuronal function through the removal of tau and amyloid beta by means of p62-induced autophagy. Future studies should help to further unravel the details of autophagy-lysosomal recycling systems in the onset and progression of Alzheimer's disease.
Top image by Jason Drees/Biodesign Institute
More Science and technology

ASU-led space telescope is ready to fly
The Star Planet Activity Research CubeSat, or SPARCS, a small space telescope that will monitor the flares and sunspot activity of low-mass stars, has now passed its pre-shipment review by NASA.…

ASU at the heart of the state's revitalized microelectronics industry
A stronger local economy, more reliable technology, and a future where our computers and devices do the impossible: that’s the transformation ASU is driving through its microelectronics research…

Breakthrough copper alloy achieves unprecedented high-temperature performance
A team of researchers from Arizona State University, the U.S. Army Research Laboratory, Lehigh University and Louisiana State University has developed a groundbreaking high-temperature copper alloy…