High-speed photography can capture a horse’s gallop, a falling star or even a speeding bullet. But such methods would be far too slow to record the elusive movements of protein molecules as they undergo transitions from one form to another — a process known as isomerization.
In new research appearing in the journal Science, an international team of researchers used brilliant bursts of X-ray light to capture the movements of a photosensitive protein — one that enables a broad range of life forms to convert light into energy.
The research represents the most detailed picture to date of nature at work at the tiniest scale. Results of the new study could pave the way for innovations ranging from renewable energy to a new generation of bio-inspired sensing devices. Similar investigations of protein structure using pioneering techniques in high-speed X-ray crystallography could also usher in a suite of new drugs, targeting a range of human diseases.
Marius Schmidt from the University of Wisconsin-Milwaukee and his colleagues led the research effort. The group was joined by researchers from the Stanford Linear Accelerator (SLAC), where the experiments were performed in collaboration with multiple institutions from around the world.
Researchers at Arizona State University’s Biodesign Institute contributed to the data analysis of the experiments, which tracked the subtle contortions of photosensitive yellow protein (PYP) over an astonishingly short time frame, running from 100 femtosecondsA femtosecond is one quadrillionth of a second. A picosecond is one trillionth of a second. to 3 picoseconds.
“Once the PYP protein absorbs a photon of light, it changes its shape from an initial configuration known as the trans form to a new shape known as cis,” said Petra Fromme, director of the Biodesign Center for Applied Structural Discovery. “The trans to cis transition occurs in such a unbelievably brief time span that nobody had been able to see the important details of this process — until our discovery.”
To give a sense of the brevity of the X-ray laser pulses illuminating the protein, there are as many femtoseconds in one second as there are seconds in 32 million years.
Light moves
Many organisms have developed sophisticated means to detect and respond to photons of light, through changes in protein structure initiated by photon absorption. The star attraction for the XFEL (X-ray Free-Electron Laser) study is PYP — a particular protein found in purple bacteria, used for sensing and responding to blue light. PYP works much like photosensors in the human eye, though the chemicals involved are different.
Photoreceptors of various kinds occur in plants, algae and fungi as well as in bacteria. Among their functions, photoreceptors help organisms reorient themselves toward or away from light, which is useful for protection against damaging high-energy light as well for maximizing sunlight exposure to grow using photosynthesis.
Scientists hope to learn more about the dynamics of biological substances, including proteins like PYP, but many have been difficult to study with conventional X-ray crystallography. The process involves crystalizing a sample of interest, then striking the crystals with a stream of X-rays, producing diffraction patterns, which are then assembled into a working structure of the protein in question. For many proteins however, growing crystals of sufficient size is a severe challenge.
Sea change
A revolution in protein imaging is now underway thanks to XFELs, which are devices that produce intense X-rays suitable for use with very tiny crystals.
XFELs yield their riches in the form of diffraction patterns before the X-ray pulse obliterates them. The method is sometimes referred to as diffract-and-destroy. Shorter X-ray pulses with greater intensity offer improved structural information.
The extremely short durations of the brilliant X-ray pulses, produced at the Stanford Linear Accelerator’s XFEL, are necessary to capture the fleeting oscillations of the PYP photoreceptor, but also provide other critical advantages, allowing tiny microcrystals to be used, rather than much larger crystals common to traditional X-ray crystallography.
Unlike earlier methods requiring crystalized samples to be kept at a chilly minus-173 degrees Celsius, the photosensitive protein under study was maintained at room temperature, more closely resembling its natural environment.
The powerful, femtosecond X-ray pulses produced by Stanford’s Linac Coherent Light Source (LCLS) are a billion times more brilliant than X-rays produced by synchrotrons. These highly energetic bursts actually destroy the crystals under observation, but the pulses are so short that diffraction images used to determine structure can be gathered before the sample is vaporized.
Essentially, the femtosecond pulses outrun radiation damage. By assembling multiple images, researchers can make detailed movies of biological molecules in motion.
“With an exposure time of about 200 femtoseconds, this is surely the fastest camera in the world. And the process we capture is closely similar to the first event in human vision, when a light photon strikes the back of your eye,” said ASU physicist John Spence, a contributor to the new study.
Capturing the movements of life
In previous studies, the researchers showed that serial femtosecond crystallography could successfully monitor the PYP structure in nanosecond-to-microsecond time scales (a thousandth to a millionth of a second). The new technique was used to reveal the fine details of cis to trans isomerization of the PYP protein in real time; in particular, its movements during the critical transition from 100 to 3000 femtoseconds.
In the current study, researchers prepared crystallized samples of PYP just 2 millionths of a meter in length, exposing each to blue laser light before injecting them into the LCLS X-ray beam. The trans to cis isomerization was found to occur roughly 500 femtoseconds after the protein’s absorption of light, with the initial transition taking place after roughly 250 femtoseconds.
In the dark, the PYP chromophore or antenna is in the trans condition and the protein is tightly folded. Absorption of a blue photon turns the chromophore yellow and induces isomerization to the cis state, in which the protein partly unfolds. The process is reversible, with the protein reverting to its folded trans state when blue light is removed.
As the authors note, the transition is marked by physical extremes, with the light-sensitive chromophore accelerating by 2 x 10 to the 15th m/s2, with a final velocity reaching 500 m/s (over 1100 mph).
Detailed studies of PYP dynamics open the door to explorations of a dizzying array of more complex proteins vital to life processes, observed at ultrafast timescales.
ASU researchers on the project are all members of the Biodesign Center for Applied Structural Discovery with appointments in the School of Molecular Sciences or Department of Physics, including faculty Petra Fromme, John Spence, Raimund Fromme, Nadia Zatsepin, Uwe Weierstall and graduate students Shibom Basu, Chelsie Conrad, Shatabdi Roy-Chowdhury, Jesse Coe, Gihan Ketawala and Ganesh Subramanian, Daniel James.
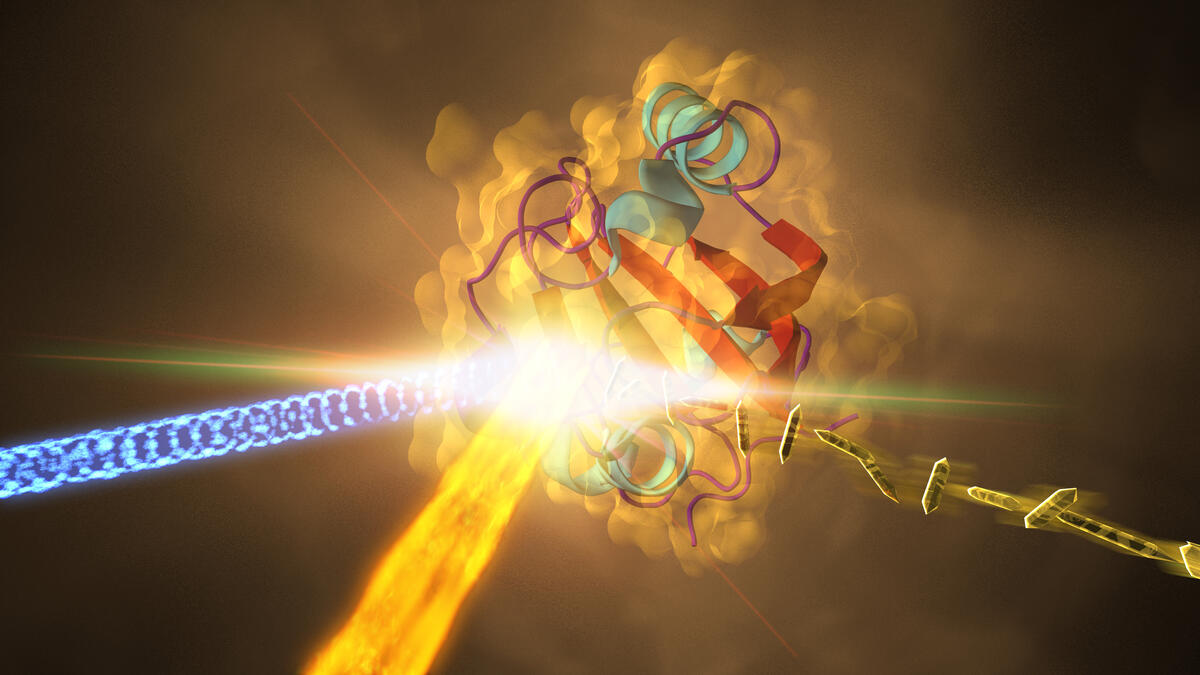
Top image: This illustration depicts an experiment at the Stanford Linear Accelerator (SLAC) that revealed how a protein from photosynthetic bacteria changes shape in response to light in less than a trillionth of a second. Samples of the crystallized protein (right), called photoactive yellow protein, or PYP, were struck by an optical laser beam (blue light coming from left) that triggers shape changes in the protein. These were then probed with a powerful X-ray beam (fiery beam from bottom left) from SLAC’s Linac Coherent Light Source. Image by SLAC National Accelerator Laboratory
More Science and technology

ASU-led space telescope is ready to fly
The Star Planet Activity Research CubeSat, or SPARCS, a small space telescope that will monitor the flares and sunspot activity of low-mass stars, has now passed its pre-shipment review by NASA.…

ASU at the heart of the state's revitalized microelectronics industry
A stronger local economy, more reliable technology, and a future where our computers and devices do the impossible: that’s the transformation ASU is driving through its microelectronics research…

Breakthrough copper alloy achieves unprecedented high-temperature performance
A team of researchers from Arizona State University, the U.S. Army Research Laboratory, Lehigh University and Louisiana State University has developed a groundbreaking high-temperature copper alloy…