When time doesn't heal all wounds
Jordan Yaron, an assistant research professor in the Ira A. Fulton Schools of Engineering at Arizona State University, is exploring new methods to promote wound healing when the normal healing process is negatively affected by diabetes and other conditions. His research has recently been supported by the Wound Healing Foundation and the National Institutes of Health. Photo courtesy Shutterstock
Many wounds aren’t a big deal — generally, all you need to do is clean them, apply bandages and let them heal. However, chronic wounds that don’t heal on their own affect more than 8 million people in the United States and represent more than $20 billion in management costs each year.
Chronic wounds are exacerbated by infection, obesity, aging and other factors. They also increase the risk of amputation and mortality for people with diabetes, which affects one in 10 Americans, with one in three currently experiencing pre-diabetes.
“These seemingly simple injuries thus pose a substantial, imminent health risk,” says Jordan Yaron, an assistant research professor in the Ira A. Fulton Schools of Engineering at Arizona State University. “Yet, we are struggling to get a handle on poorly healing wounds. I want to determine how we turn a chronic, non-healing wound into a healthy, healing wound.”
Yaron’s research focuses on understanding the complex signals involved in healthy wound healing. By identifying which of these signals are dysfunctional in poorly healing wounds, scientists could develop therapeutic approaches for reversing these signals to enhance healing.
He is taking a different approach than the therapeutic norm, which includes using techniques like delivering growth factors, dressings and matrices to attempt to force wound closure.
“This hasn’t been as successful as expected because wound healing involves a complex, overlapping sequence of phases that are independently receptive to different treatments,” Yaron says. “That is to say, an effective early stage healing treatment may be ineffective in later stages, and vice versa. Acute wounds can convert to chronic wounds anywhere along the healing continuum.”
Jordan Yaron
Instead, Yaron’s approach is to investigate the normal behaviors of a healthy wound, whether they are altered in a poorly healing wound and if the momentum can be pushed back toward healthy healing by correcting the dysregulated behaviors.
“This led to the surprising finding that skin turns on a pathway after being wounded that has seemingly no prior known connection to tissue growth, but nonetheless appears to be important in healing,” says Yaron, who is a faculty member in the School for Engineering of Matter, Transport and Energy, one of the seven Fulton Schools at ASU.
Yaron’s work in this field has earned recent attention from both the Wound Healing Foundation and the National Institutes of Health.
Helping the body heal itself
Yaron was selected as the 2022 Wound Healing Foundation 3M Fellowship award recipient for research into wound healing regulation mediated by epidermal serpin, a type of protein. The highly competitive fellowship is the most prestigious young investigator grant awarded by the foundation and supports only one researcher annually from a U.S. university or medical school.
“The Wound Healing Foundation is an essential force in the effort to promote research, education and outreach for wound healing in the U.S. and internationally,” Yaron says. “To be selected as the 2022 recipient of the fellowship means to me that the innovative research we’re doing to develop next-generation therapeutics and drug delivery platforms at ASU is recognized as a promising and important contribution to the wider effort to improve outcomes for patients dealing with complicated wounds.”
The term “serpin” is an abbreviation of the name for a superfamily of proteins called serine protease inhibitors. They are found in every form of life, from humans to mice to fungi to viruses and everything in between.
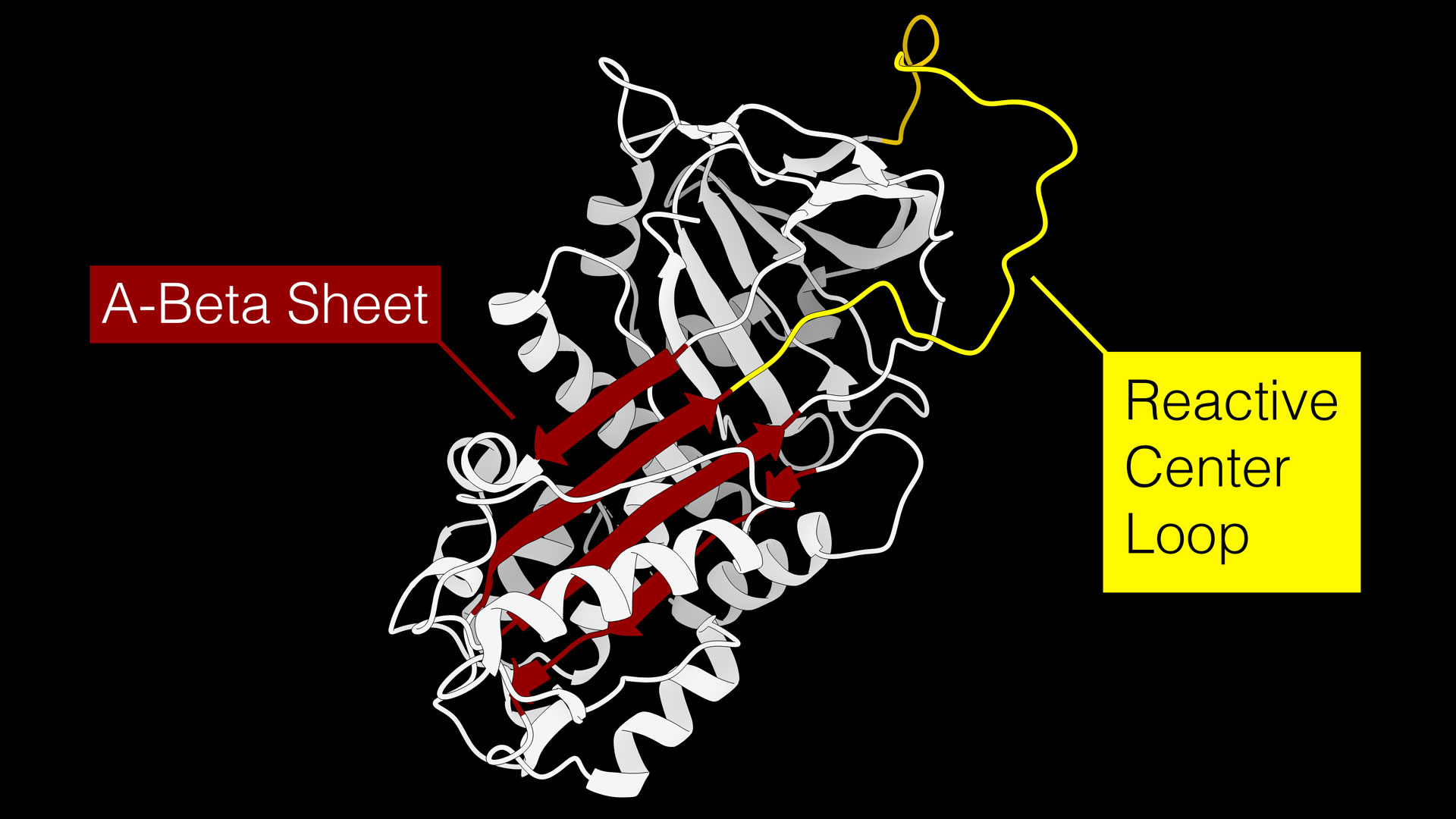
A figure showing a serine protease inhibitor, or serpin. Serpins regulate a wide variety of biological processes by disabling activated proteases, such as those that might impose a barrier to healing in the first few days of the healing process by functioning similar to a mousetrap. The reactive center loop, shown in yellow, acts as bait to attract proteases that are trying to eat the serpin. When the protease goes to eat the serpin’s reactive center loop bait, it swings down into the A-Beta Sheet, shown in red, and disables both the protease and the serpin. Graphic courtesy Jordan Yaron
“In essence, serpins are molecular mousetraps,” Yaron says.
A loop that extends from the serpin acts as “bait” for target proteases, which are enzymes that eat and break down proteins. During the early stages of wound healing, proteases are important participants of the process because they break down damaged proteins and remodel the wound environment to allow pro-healing cells to migrate.
“Impaired wounds are often found to contain an overabundance of proteases, which may also act to inappropriately break down helpful proteins,” Yaron says. “Controlling the levels of activated proteases in a wound may help to resolve barriers to healing.”
The serpin “mousetrap” is a mechanism by which protease levels can be regulated. When a protease goes to eat the serpin protein, the mousetrap snaps shut and permanently disables both the protease and the serpin.
“These remarkable machines are surprisingly understudied relative to other classes of protein in our body,” Yaron says. “Our genome codes for 37 different serpins, and they participate in regulating hundreds of processes in our body. They account for up to 10% of the circulating proteins in our blood.”
Yaron’s research team identified one serpin that is absent in healthy skin, but is suddenly and abundantly expressed as soon as skin is wounded. Levels of this serpin increase for the first few days of healing, and then it is turned off again.
“In diabetic wounds, this serpin has a hard time turning on,” Yaron says. “We found that when we recombinantly synthesized this serpin and delivered it like a drug, it accelerated wound closure in both cell cultures and mice. All we’re doing is putting a protein there that should already be there, but which struggles to show up in a diabetic environment.”
For the Wound Healing Foundation 3M Fellowship, Yaron will explore the fundamental mechanisms by which serpins regulate wound healing, and he hopes to uncover ways to enhance their healing properties.
Yaron says he believes he was selected for the fellowship, which provides $15,000 in funding, due to the importance of finding a new mediator for the healing process and the potential to harness the serpin protein to open new methods for developing therapeutics to treat poorly healing wounds.
And he’s proud to bring the award to ASU.
“I’m a four-time Sun Devil — I received my Bachelor of Science and PhD, completed my second post-doctoral training and I am now an assistant research professor, all at ASU,” Yaron says. “It gives me tremendous pride to represent the ASU community and to bring this recognition home.”
Borrowing from nature to bioengineer new treatments
Yaron also explores how to use natural materials not already found in the human body to promote wound healing. In 2021, he was awarded a three-year, $308,954 K01 Mentored Research Scientist Career Development Award by the National Institutes of Health to explore a method to augment tissue repair through engineered biomaterials.
Diabetes causes poor conditions for healing in wounds, including damaged blood vessels that result in limited blood flow, biochemical imbalances and dysregulated biological mechanisms of tissue repair. In addition to limiting natural healing, these conditions also limit the effectiveness of therapeutic treatments. Burns and infections can also hinder healing and treatment delivery. So, new engineered biomaterials are needed to deliver treatments that promote healing in these types of wounds.
In this research, Yaron is exploring the use of silk fibroin from domestic silkworm cocoons and chitosan, a type of sugar, from shrimp and other shellfish shells for a timed, sustained-release method of delivering treatments for wound healing.
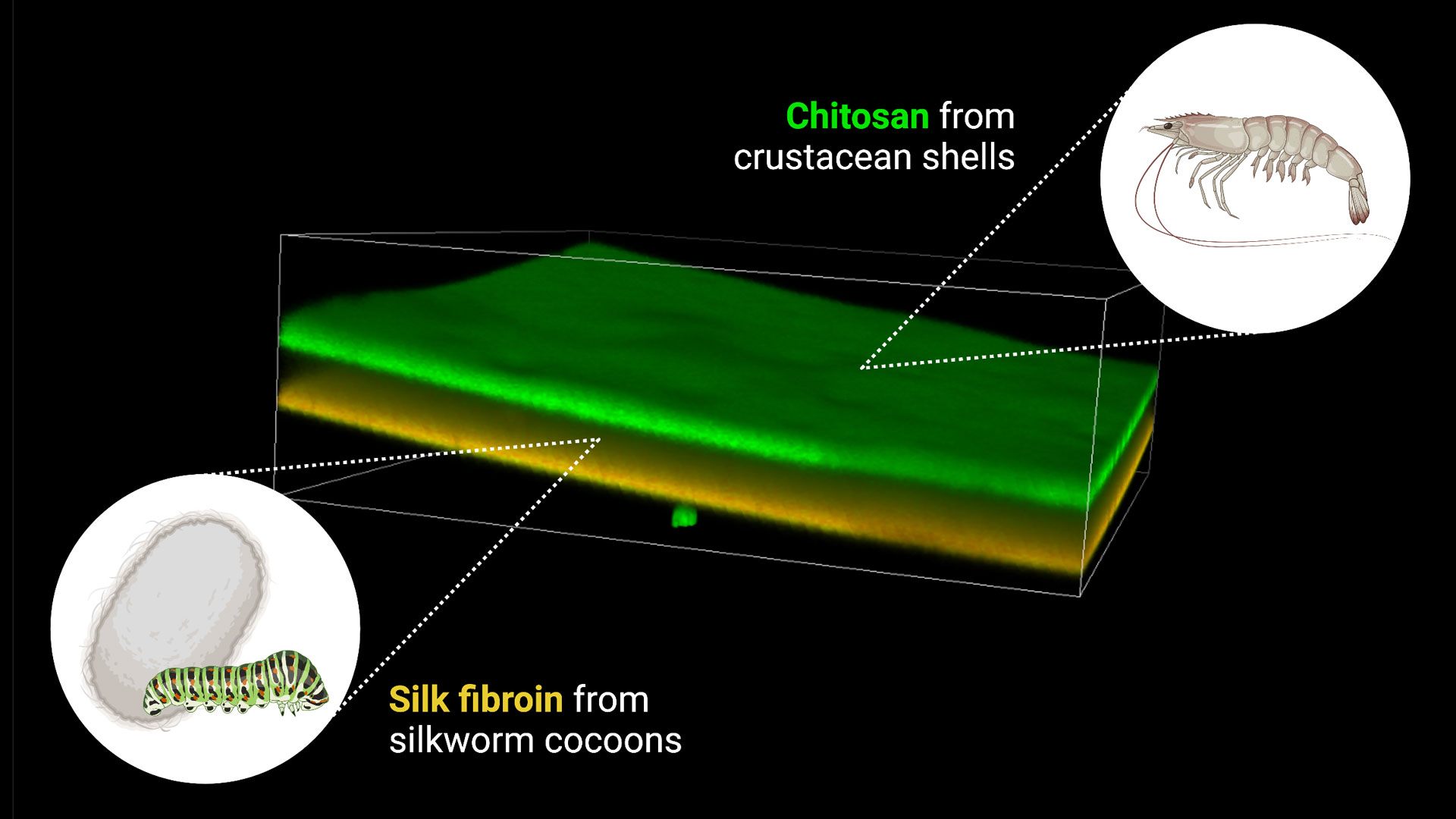
A 3D confocal laser microscopy image of a composite bilayer film generated from chitosan from crustacean shells (dyed green) and silk fibroin from silkworm cocoons (dyed orange). The film is less than 200 micrometers thick. The two layers of the film have different drug release and degradation properties and can be used to generate a “smart wound dressing” that can release one drug quickly and another drug slowly, timed to the rate of wound healing. Image courtesy Jordan Yaron
“We chose these materials because they are natural polymers with very interesting biological and physicochemical properties,” Yaron says.
Silk fibroin is mechanically tunable, compatible with living tissue and the researchers can control the rate at which it breaks down. The lab Yaron works within — the Rege Bioengineering Lab directed by Kaushal Rege, a professor of chemical engineering in the Fulton Schools — has also developed protocols to modify silk fibroin to further adjust its properties.
Chitosan is also compatible with living tissue, as well as biodegradable, and can easily be modified to change its chemical properties.
By leveraging the properties of each of these materials, Yaron and Rege Bioengineering Lab researchers are building a library, or toolkit, to load and release different drugs, composed of small molecules and proteins, at different rates from a composite material. They will tune the biology and physical chemistry of silk fibroin and chitosan to create composites and find combinations that optimally interact with healing wounds.
“Our composite drug delivery platforms may have emergent properties that cannot be entirely predicted. By building several libraries of materials, we can screen a large set and identify the platform that exhibits the properties we have identified as necessary to best aid in wound healing,” Yaron says. “Our goal is to make a single ‘smart wound dressing’ that can release one drug quickly and another drug slowly with the goal to time the exposure of the drug to the phase of healing.”
Yaron plans for the composite to carry drugs that will address different phases of the wound healing process, particularly transitioning from the inflammatory phase to the proliferation phase to wound closure. Diabetic wounds tend to have difficulty transitioning through these phases.
First, he aims to deliver small molecule inhibitors of the inflammasome, an immune complex that drives inflammation and is important to healthy wound healing but dysfunctional in diabetic wounds.
Then, a few days into the healing process, the composite would release growth factor nanoparticles to help push the wound into the next phases of healing — proliferation and then closure.
“It’s like pushing a car up a hill,” Yaron says. “If a wound has a hard time getting to the top of the hill, the growth factor nanoparticles will get it there, and then the wound should have less of a hard time progressing to closure, or going down the hill.”
If his research is successful, Yaron hopes the new toolkit of smart dressings with a variety of bioactive cargo options will maximize our ability to “harness the healing process” and be used in a clinical setting. Because both naturally derived materials are Food and Drug Administration-approved and Generally Recognized as Safe materials, the composite package of this treatment method should be easy to gain approval. However, there is still a long road to getting the bioactive cargo through FDA approval to clinical application.
“Translating from the bench to the bedside never happens overnight, but because we have strong science behind our molecular targets and a distinct readout goal in our preclinical models, we hope we will avoid the scenic route,” Yaron says.
As part of the K01 mentored research award, Yaron is working with Rege, his primary mentor and an expert in developing innovative biomaterials for tissue repair, including through the use of gold, silk and lasers.
To complement his expertise in cellular inflammation and tissue repair therapeutics development, he is also working with a strong team of mentors who have expertise in bioengineered nanomaterials, dermatopathology, biomaterial immune responses and translational therapeutics: Francois Berthiaume at Rutgers University, David J. DiCaudo at Mayo Clinic, and Tatiana Ugarova and Alexandra Lucas at ASU.
“Working with Dr. Rege and the rest of my mentorship team in the innovative and scientifically robust environment at ASU provides an outstanding opportunity to bridge their worlds with mine and to hopefully emerge with something truly novel and impactful to the wound healing community,” he says.
More Science and technology

ASU and Deca Technologies selected to lead $100M SHIELD USA project to strengthen U.S. semiconductor packaging capabilities
The National Institute of Standards and Technology — part of the U.S. Department of Commerce — announced today that it plans to…
From food crops to cancer clinics: Lessons in extermination resistance
Just as crop-devouring insects evolve to resist pesticides, cancer cells can increase their lethality by developing resistance to…

ASU professor wins NIH Director’s New Innovator Award for research linking gene function to brain structure
Life experiences alter us in many ways, including how we act and our mental and physical health. What we go through can even…