New review highlights cancer-crushing viruses

Biodesign Center for Immunotherapy, Vaccines and Virotherapy researchers Grant McFadden (left) and Masmudur Rahman.
With the world still in the grip of a devastating pandemic, it’s hard to imagine viruses as something other than hostile enemies to be vanquished.
But in a recent review article for the journal Cancers, Masmudur Rahman and Grant McFadden describe a class of viruses that act to combat rather than cause deadly disease. Such oncolytic viruses, as they are known, have a remarkable ability to target and destroy cancer cells while leaving healthy cells untouched.
“The field of oncolytic virotherapy today is advancing rapidly as clinical trial data accumulates and regulatory approvals continue to accrue,” said Rahman.
Rahman is a researcher in the Biodesign Center for Immunotherapy, Vaccines and Virotherapy. McFadden, a pioneer in the field of oncolytic viruses, directs the center.
Viral universe
By a wide margin, viruses are the most abundant biological entities on Earth, easily outnumbering all other life forms combined, though they inhabit a shadowy world somewhere between living and inanimate matter.
Viruses infect every form of cellular life, including animals, plants, bacteria and fungi. While they are notorious for causing serious illness, they also play vital roles in evolving ecosystems — phenomena scientists are only beginning to appreciate.
Viruses can roughly be broken down into “specialists,” which are selective in the particular organisms they infect, and “generalists,” which are more promiscuous about the species they target and invade. Oncolytic viruses lean toward the specialist category. While showing little to no danger for normal mammalian cells, they can be fierce assassins of the malignant cells associated with cancer.
Cancer remains a leading killer globally and is anticipated to cause 1.9 million cases and 608,570 fatalities in 2021 in the U.S. alone, according to the American Cancer Society. The discovery of cancer-killing or oncolytic viruses has opened a new door on cancer therapies that may fulfill the elusive goal of eradicating cancer while leaving healthy cells and tissues unharmed.
Some of the key advantages of oncolytic viruses as well as strategies to improve them are seen graphically below.
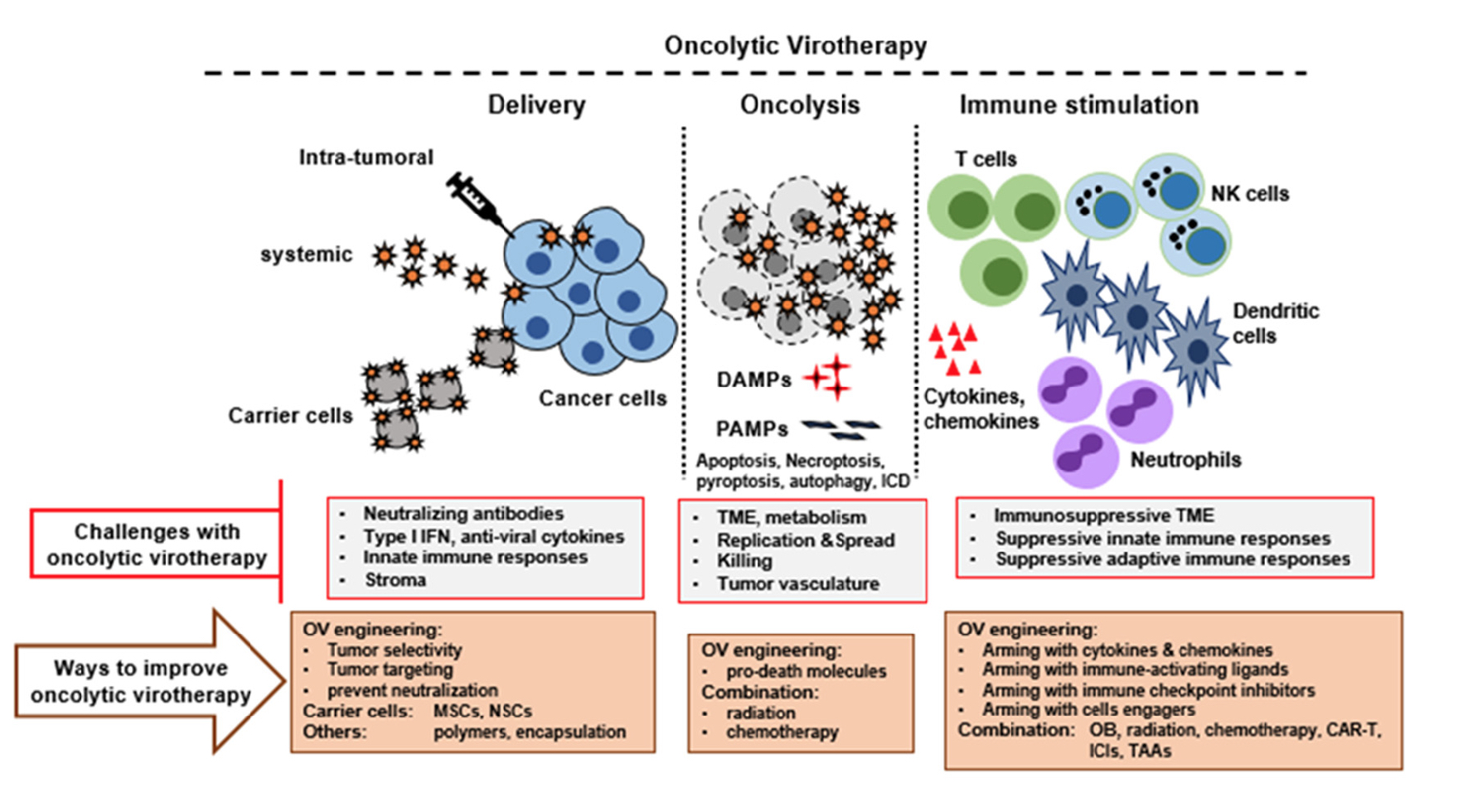
This graphic outlines the process of introducing oncolytic (cancer-killing) viruses. They may be injected directly into tumors or transported to the affected site with carrier cells. The viruses then begin to attack and kill cancer cells and trigger cell suicide processes like apoptosis and necroptosis. The oncolytic viruses then act to stimulate various cells of the immune system, alerting them to the presence of viral invaders, as well as cancer, and helping them overcome cancer's ability to suppress immune activity. The graphic also shows a number of tactics for improving oncolytic viruses. Graphic courtesy of the journal Cancers
Natural-born killers
The first clues that oncolytic viruses may exist in nature came more than a century ago, when physicians noticed that some cancers appeared to regress in patients that also had microbial infections. For example, near the end of the 19th century, a leukemia patient was reported to regress under the influence of a flu-like disease accompanied by inflammation, directly implicating viruses as cancer-thwarting entities.
Today, a variety of oncolytic viruses are being explored for cancer therapy. While many such viruses can directly attack and terminate malignant cells, their primary strength may lie in their ability to alert an inactive or disabled immune system to the presence of cancer.
When successful, the oncolytic virus triggers the patient’s own cellular sentry dogs of immunity to seek out cancer and destroy it, as they would a foreign pathogen.
Researchers like McFadden and Rahman have learned to reengineer oncolytic viruses to sharpen their lethality to cancer cells as well as their ability to stimulate the immune system. Two primary methods are used in such viral gene engineering, known as knockout and knock-in approaches.
Knockout refers to the removal of viral genes, prior to introducing the virus into patients. Knock-in methods involve the introduction of novel genes, known as transgenes, into naturally occurring oncolytic viruses.
Building better oncolytic viruses
At the Biodesign Center, McFadden, Rahman and their colleagues work with myxoma virus, an oncolytic virus that shows considerable promise. Myxoma is a member of a large family of viruses known as poxviruses. Myxoma is nonpathogenic to humans but it’s close to 100% lethal to European rabbits, producing an aggressive disease known as myxomatosis. When introduced into a bed of cancer cells, myxoma attacks and kills them. Yet the virus is harmless to non-cancerous human cells (or those of any other non-rabbit species).
Myxoma is a double-stranded DNA poxvirus. Like most others in this vast virus group, it has a relatively large genome, made up of over 160,000 genetic base pairs, which code for 171 viral genes.
One reason myxoma is an attractive candidate for oncolytic virotherapy is that its large genome is amenable to improvements using genetic modification. Alterations involving transgenes can further enhance the ability of oncolytic viruses to recruit immune system cells to recognize and target the tumor, turning hidden or "immune-cold" cancers into "immune-hot" cancers vulnerable to destruction.
Viral transit
One of the challenges for oncolytic virotherapy is getting the viruses to the affected cancer region. In some cases, the viruses can be injected directly into the cancer site. But for cancers hidden in organs or tissues within the body, the viruses need to be escorted through the bloodstream to the proper site. This can be accomplished by using carrier cells to transport the virus to the cancer.
Many types of carrier cells are being explored for this purpose. Sometimes the oncolytic virus is embedded within the migratory carrier cell’s interior after the virus has infected the cells. In other cases, the oncolytic viruses are attached to carrier cell surfaces. In either case, the virus now has a means to migrate to the cancer, attacking it and stimulating the immune system.
Different classes of carrier cells are appropriate for different oncolytic viruses and cancer types to be treated. Among the many carrier cells under active investigation are patient‐derived mesenchymal stem cells (MSCs), which may enhance the anti-tumor properties of oncolytic viruses.
Therapy goes viral
The full potential of oncolytic viruses will almost certainly involve combining them with existing cancer treatments like radiation, chemotherapy and various forms of immunotherapy. One of the most promising new treatments is a form of immunotherapy using so-called checkpoint inhibitors.
Checkpoint proteins are produced by the immune system’s T cells. These regulatory agents act to prevent an overresponse by the immune system, which over time can damage healthy cells and tissues. Cancer often exploits this system, making use of checkpoint proteins to protect themselves from immune assault.
Checkpoint-inhibiting drugs can switch the immune system back on, allowing T cells and other immune components to vigorously attack the cancer.
“When patients respond to checkpoint inhibitors, it’s often like magic,” McFadden says. “They can undergo massive tumor regression and long-term, disease-free survival.”
Rahman agrees, with a caveat: “New approaches like immune checkpoint inhibitors are very effective in reducing the tumor burden and improving survival, but it works only in a small number of patients — only 10–20%.”
Describing the mysterious disparity between responders and non-responders, the authors suggest that patients whose cancer responds to the drug may have mounted an initial immune response to cancer that was suppressed. In these cases, removing the suppression with checkpoint inhibitors can reactivate the immune system to do its job.
In contrast, patients whose cancer doesn’t respond to checkpoint inhibitors may never have developed a proper immune response, leaving nothing for the checkpoint inhibitor to restart.
“We believe that if you get an infection going in the tumor bed, you can generate a new kind of immune response to both the tumor and the virus at the same time,” McFadden says. “That new immune response, we hope, will result in more patients being able to benefit from immunotherapies like checkpoint inhibitors.”
While only four oncolytic viruses have thus far been approved for global use, many more — including myxoma — are in pre-clinical trials as the field rapidly advances. McFadden has formed the company OncoMyx Therapeutics to advance myxoma’s cancer-combating potential into clinical trials.
More Science and technology

Hidden viruses thrive in desert wildlife
As the sun rises over the Sonoran Desert, bright green lovebirds gather noisily around backyard feeders. At dusk in the Arizona…

2 ASU faculty named NAI fellows
Professors Krishnendu Chakrabarty and Rosa Krajmalnik-Brown are among the latest Arizona State University faculty members to be…

How AI is changing college
Artificial intelligence is the “great equalizer,” in the words of ASU President Michael Crow.It’s compelled industries, including…