The ancient Greek philosopher and historian Plutarch once wrote, "Medicine to produce health must examine disease.” And for the generations upon generations of physicians since Plutarch’s time, medicine has focused on treatments by first examining the symptoms of disease.
The annals of medicine are ripe with descriptions of “poxes,” “the cholic,” or even the sudden loss of smell or taste with the current global pandemic.
But now, a new movement has been afoot that examines disease based entirely on the molecular profiles of disease. In study after study, scientists examine whole genetic profiles in organs, tissues and even down to the level of molecules within individual cells in the hopes of teasing apart the mysteries of intractable diseases that are the leading causes of death throughout the world.
Called personalized medicine (or precision medicine), this movement uses genetic or other biomarker information found in blood, urine, spit, stool — you name it — to make the best treatment decisions for each individual patient. Now, physicians talk about breast cancer patients being HER2+, triple negative and examine their TP53 mutational profile. Or, with COVID-19, saliva tests and mRNA-based vaccines help thwart delta variants of SARS-CoV-2.
And with the increased scrutiny of these molecular disease profiles, comes the power of possibly predicting or diagnosing diseases at their earliest stages. Or better yet, customizing therapies that can thwart disease before it’s too late to turn back time. It’s the simple idea of an ounce of prevention leading to a pound of cure.
Back to the start
David Brafman
Arizona State University Associate Professor David Brafman, a stem cell expert, wants to use this new knowledge and tweak it with a slightly different approach, to take the best of these molecular clues and trace them back all the way back to the start — to catch the very first manifestations of a disease.
"In terms of motivation, I more or less fell into the Alzheimer's disease research field about 10 years ago," Brafman said. "However, once I did, it became apparent to me what a devastating disease it is and the impact it has not only on the patients but also the caregivers."
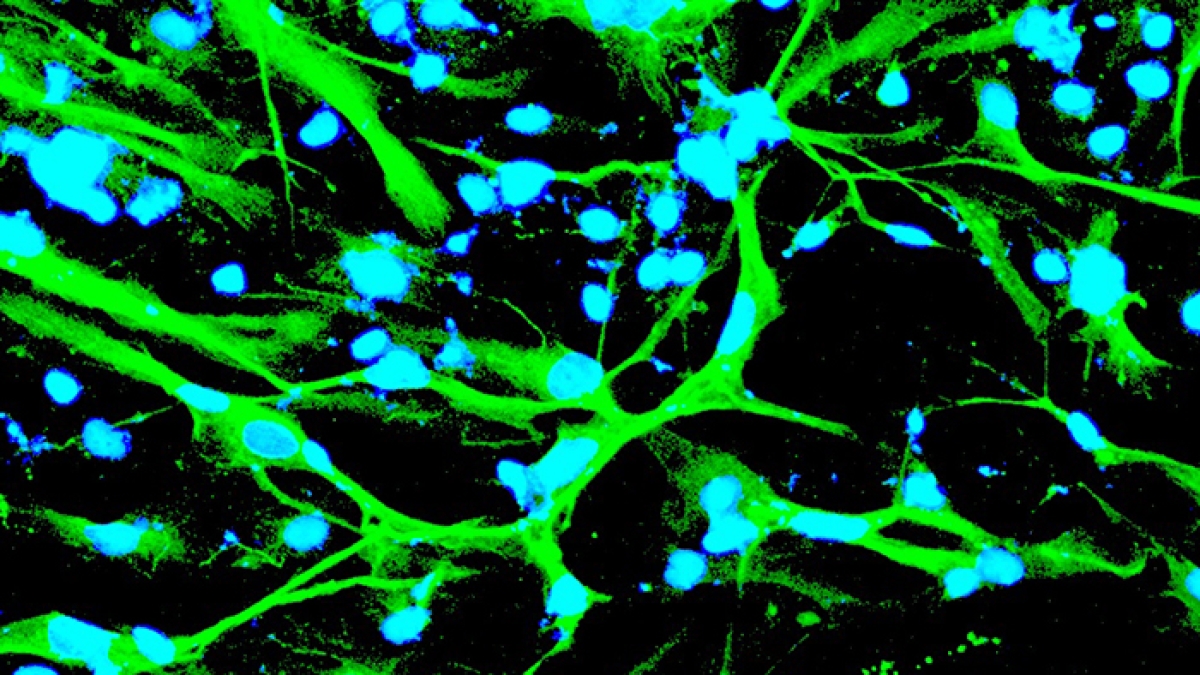
His scientific search for a cure is to reprogram stem cells, by cutting and pasting replacement genes, and the protein molecules they make, into stem cells. In some cases, they may have a beneficial effect. In others, normal cell functions may suddenly go haywire. Either way, his lab watches for the first telltale signs of the effects these replacement molecules may have within stem cells, as they divide and multiply into possibly damaged, cells, tissues and organs.
“Our laboratory uses pluripotent stem cells as a model system to investigate various aspects of neurodegenerative disease, with a focus on Alzheimer’s disease. Our hope is that, with this model system, we will not only understand the underpinnings of the disease but also design and test more effective therapies,” said Brafman, a professor in the School of Biological and Health Systems Engineering, one of the ASU Ira A. Fulton Schools of Engineering.
Traditionally, the field of regenerative medicine has been associated with cell replacement therapies in which tissues or organs have been damaged by disease, trauma or congenital issues by using the seemingly unlimited potential of stem cells for tissue engineering, cellular therapies or, perhaps one day, artificial organs grown from a patient’s own genetically reprogrammed stem cells.
Brafman’s laboratory, on the other hand, uses the potential of these cells to generate cells and tissues outside of the body to better understand what is occurring with diseases that occur within the body.
And now, in a pair of recent experiments, Brafman’s team has, for the first time, developed a proof-of-concept new lab tool that's set the stage to put these his plans into motion, by looking into the role of one of the leading genetic culprits that may be at the intractable root of Alzheimer’s disease.
David Brafman's students look at a plate of growing stem cells under the microscope.
Questions of science
But how can one uncover the root of a dementia disease that slowly destroys the mind, whose symptoms of "forgetfulness" and "not able to progress in writing" were first described by German physician Alois Alzheimer's examination of a housewife 100 years ago?
In his detailed case history of a far-from-elderly 51-year-old Frankfurt native, Auguste Deter, she repeated a phrase that haunted him during his examinations: "I have lost myself."
It's a sentiment ghostly echoed in time among the 6 million Americans currently living with Alzheimer's disease today.
For despite scientists' Plutarchian progress and Herculean efforts examining Alzheimer's disease, today there is still no cure. The scientific road ahead still seems Sisyphean. A sentiment that Coldplay frontman Chris Martin may have expressed best in the band’s hit song “The Scientist": "Nobody said it was easy."
Since 2002, nearly 100% of Alzheimer's disease clinical trials have failed.
Just two years ago, the FDA-approved Aduhelm (aducabanad), hailed as the first new Alzheimer's disease drug in a generation, has been met with skepticism and disapppointment by the medical community. That’s because Alzheimer's is a disease that is decades in the making. Aduhelm may simply be a case of too little, too late.
Even by the time symptoms first appear, the damage done to the human brain may already be too late to reverse.
Upon her death in 1906, Deter's brain was examined by Alzheimer. He could see for the first time, her behavorial symptoms appreared to physically manifest themselves as brown-stained plaques within the meticulously thin slices made from her preserved brain. Nowadays, scientists identify these plaques as filled with the detritus of Greek-named fragments from protein molecules called amyloid-beta (Aβ) or tau.
The only known risk factor is family history. So, a quarter of a century ago, scientists performed the first genetic screens to look for genes that may be passed down in families or result in the early onset of symptoms.
To date, the most promising predictor for risk of Alzheimer’s disease during one's lifetime is a gene called apolipoprotein E (APOE). APOE was known to play a key role in cholesterol metabolism. At the time, the results were surprising, because it was not clear how a gene normally associated with heart health could also affect the brain.
Further studies found that the APOE gene comes in three varieties (described scientifically as alleles). Most people have an APOE3 form of the gene. Interestingly, the APOE4 version was associated with an up to 10 times higher risk of developing Alzheimer’s disease. The third version, APOE2, was thought to play a more protective role because it was mainly found in families who usually did not develop the disease.
Brafman wanted to know: Could APOE2 be the ounce of prevention needed to begin to stem the tide on Alzheimer’s disease?
Studies that have followed individual families over a long period of time have shown that the presence of the APOE2 variant reduces the lifetime risk of developing Alzheimer's disease by 40%. That further piqued Brafman’s interest into learning more about the mysterious protective role of APOE2.
“While there has been significant research that has identified the risk-inducing effects of APOE4, the underlying mechanisms by which APOE2 influences Alzheimer’s disease onset and progression have not been extensively explored,” Brafman said. “Our studies provide new insights into the risk-modifying effects associated with the APOE2 allele and establishes a platform to probe the mechanisms by which APOE2 enhances neuroprotection against Alzheimer's disease.”
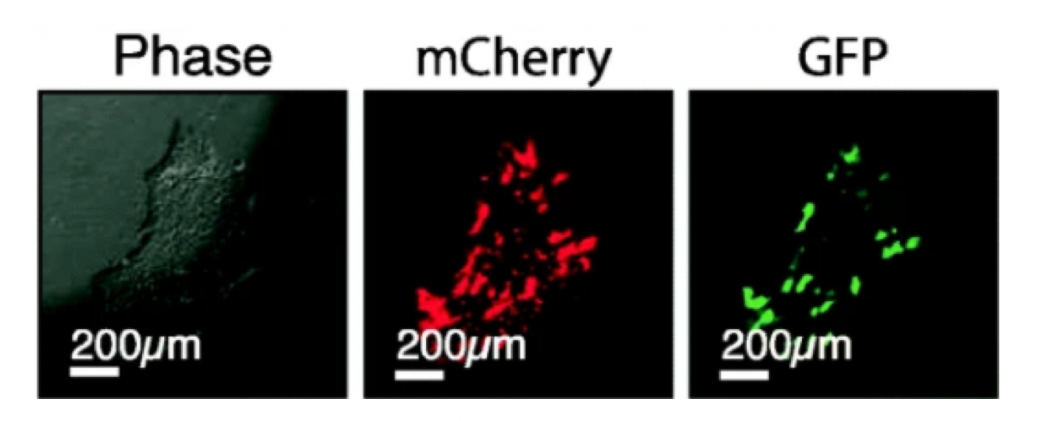
A batch of freshly grown neuronal stem cells help researchers investigate AD. On the left is an image of the cells using a phase contrast microscope, which can help visualize the cells. In the middle picture, mCherry is added to the cell to help make it glow red under fluorescent light. On the right, green fluorescent protein (GFP) is added to make them green.
Science and progress
Brafman’s approach was to replace the APOE3 version of the gene with APOE2 in stem cells, and to see whether or not they could reduce the production of the amyloid-beta (Aβ) protein fragments found in the plaques.
At the molecular level, APOE2, APOE3 and APOE4 only differ by two amino acid substitutions for the APOE proteins.
Imagine that just two tiny amino acid swaps in a protein found within a cell could make all the difference for whether Deter, or your grandma or grandpa, would be protected or not from Alzheimer's disease.
Specifically, the mutations are found at amino residues 112 and 158 within the 299 amino acids that make the APOE proteins. The APOE2 molecular profile is Cys112, Cys158, while APOE3 is Cys112, Arg158, and finally, APOE4 is Arg112, Arg158.
For instance, to make the APOE3 to APOE2 swap, Brafman makes the amino changes in the lab, using the breakthrough CRISPR technology to target and replace the gene within the DNA genome of the stem cell. CRISPR works like a pair of molecular scissors to cut out and replace genes down to single amino acid changes. Since humans have two copies of the APOE gene, he can replace one, or both, and study their various effects.
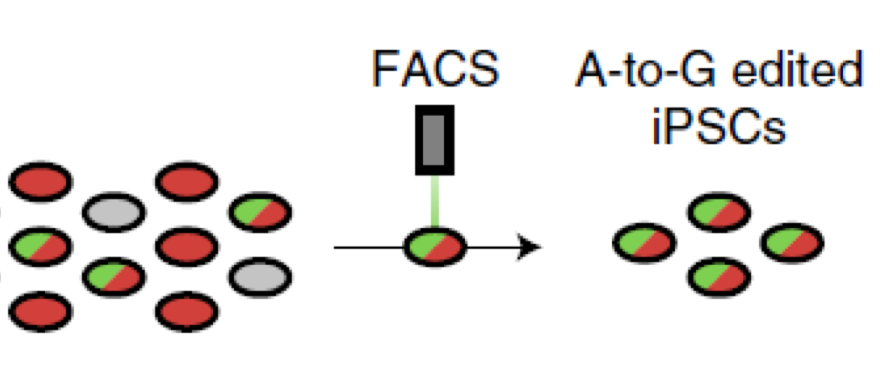
Using CRISPR technology, small edits are made to the APOE gene. Brafman's new TREE method works by tagging the APOE edited gene to give off a light signal. A FACS machine can see the light (in this case, only red and green colors within a single cell) and sort out only the stem cells that have APOE genes edited, all at efficiencies approaching 90%.
This meant that instead of all the cells containing just one form of the APOE gene, there was likely a patchwork, genetic quilt of APOE cells containing either APOE3 or APOE2. But because he wouldn’t know which cells contained which gene, Brafman would not be able to provide a crucial test for APOE2 proving cause and effect.
To carry out his studies, Brafman had to go back to the drawing board and invent a more efficient process, which he calls TREE (a transient reporter for editing enrichment).
Briefly, the TREE methods work by piggybacking a fluorescent reporter tag to the APOE edited gene that, when excited by light, gives off a signal. This allows a machine to spot the signal and sort out only the stem cells that have had the single amino acid changes made from the unedited cells. With the technology, now his research team could generate stem cell lines in just three to four weeks with both APOE genes edited at efficiencies approaching 90%.
And the beauty of Brafman’s TREE system is that it can be applied to any gene to study any cellular or disease process.
“Although our initial studies with TREE have focused on the role of APOE as it relates to AD risk, the beauty of this system is that it can be applied to the interrogation of any gene. In fact, we are currently using this system to study various of genetic factors that alter risk of AD onset and age-related progression. In addition, we have been pleased that folks studying other diseases have implemented TREE-based strategies for their work.”
Pulling the puzzles apart
In the study, the team generated a series of stem cell lines and studies that harbored known human mutations associated with early onset Alzheimer's disease with either the APOE3 gene or gene-edited APOE2.
Remarkably, when they performed their analysis of pure populations of neurons and astrocytes derived from these stem cell neural cultures, it began to reveal the protective effects of APOE2 by reducing the amount of Aβ and tau fragments deposited within the brain cells.
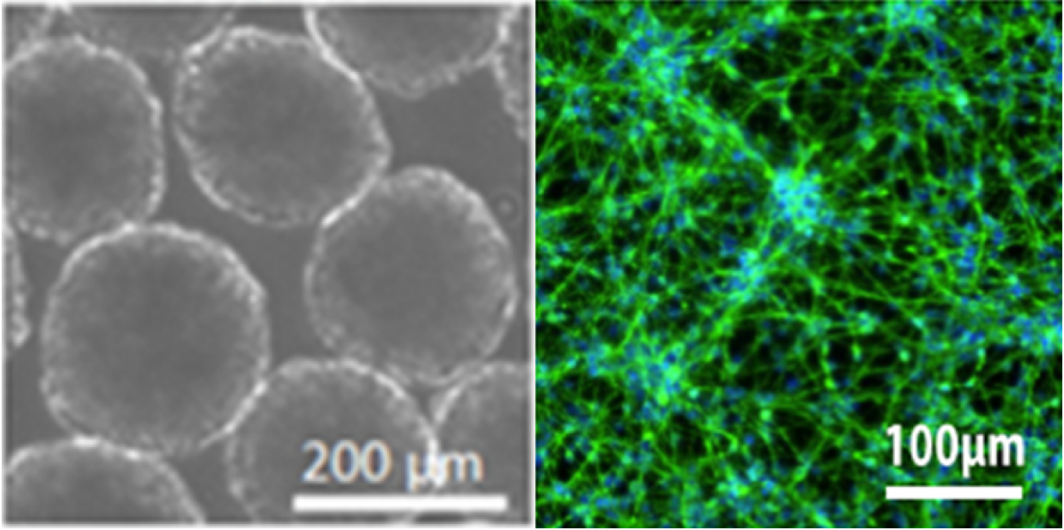
A picture of organoids (left) and neurons (right), grown in the lab from stem cells. The microscopic images are at the scale of microns, or one-thousandth of a single millimeter on a ruler.
“In particular, we demonstrated the reduction in Aβ is potentially driven by a mechanism related to non-amyloidogenic processing of amyloid precursor protein, suggesting a gain of the protective function of the APOE2 variant,” Brafman said.
In the body, APOE works to help break down fats and transport them to other cells for energy. In the central nervous system, APOE is generated and secreted mostly by supportive brain cells, called astrocytes and microglia, and, to a lesser degree, neurons themselves. In the brain, APOE functions to transport cholesterol and other lipids to neurons and plays important roles as it relates to neuronal growth, membrane repair and neural plasticity.
Brafman says it’s possible that APOE2 helps prevent Alzheimer's disease by altering the manner in which amyloid precursor protein is processed, which results in a reduction in the pathological accumulation of Aβ.
Next, his group will work in concert with colleagues at ASU and its clinical partners to continue to tease out exactly how APOE accomplishes this, by performing a Plutarchian disease examination as never before to truly understand Alzheimer's disease at the molecular level.
“We’re hopeful that understanding the mechanisms that underlie the association between APOE2 and protection against neurodegeneration may also provide new therapeutic targets for AD,” Brafman said. "My lab and I have no grand illusions that we alone will be the ones to find the cure for Alzheimer's, but what I tell my lab is as follows: Each of us have unique expertise and talents. As such, it is our obligation to contribute whatever we can towards the collective effort among all researchers and scientists who study and understand this devastating disease. If all researchers have this 'we sink or swim together' attitude, then together, finding a treatment is possible."
With his lab’s new TREE technology and continued studies, they remain hopeful that stem cell science can enter the scene to give a major boost to begin to translate their results into the clinic, for Alzheimer's and other diseases.
The leading Alzheimer's researchers in the state will gather together to share their latest results at the 2021 Arizona Alzheimer’s Consortium Conference on Thursday, Sept. 9.
More Science and technology

Google grant creates AI research paths for underserved students
Top tech companies like Google say they are eager to encourage women and members of historically underrepresented groups to consider careers in computer science research.The dawn of the era of…

Cracking the code of online computer science clubs
Experts believe that involvement in college clubs and organizations increases student retention and helps learners build valuable social relationships. There are tons of such clubs on ASU's campuses…
Consortium for Science, Policy & Outcomes celebrates 25 years
For Arizona State University's Consortium for Science, Policy & Outcomes (CSPO), recognizing the past is just as important as designing the future. The consortium marked 25 years in Washington, D…