Newly discovered leprosy genomes help fill historic gaps
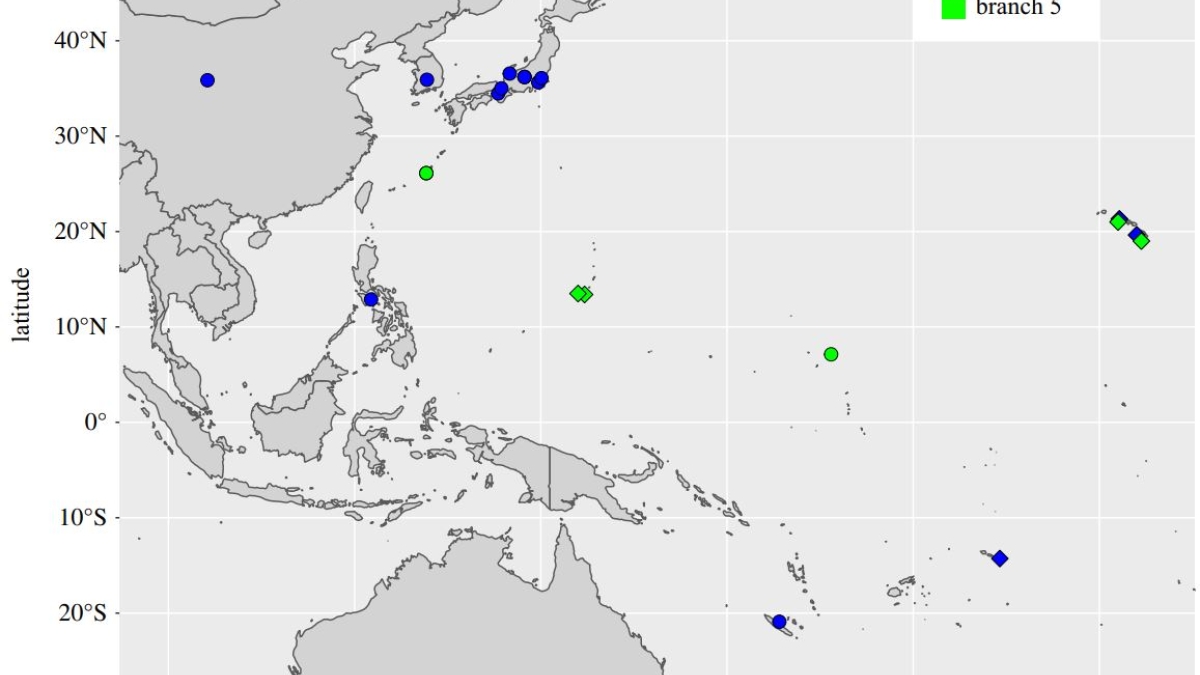
Figure 1. “Map of the Pacific showing source locations of novel and previously sequenced M. leprae strains from branch 0 and branch 5. Novel strains from this study all fall within these branches and are shown as diamonds,” the researchers wrote. “Comparative data are shown as circles.” Strains of M. leprae have geographical associations. The newly discovered genomes belonged to strain 0, which is predominantly found in East Asia, and branch 5, which is largely the Pacific Islands. Reprinted with permission from the Philosophical Transactions B journal.
History books often link disease and colonization, in which explorers unknowingly bring with them illnesses that spread to native people. New findings on the origin of leprosy in the Pacific Islands, however, contradict this suggestion.
Arizona State University graduate students Kelly Blevins and Adele Crane are the primary co-authors of a study identifying nine new genomes of Mycobacterium leprae, one of the two pathogens that cause leprosy. Previously, only two other genomes of M. leprae had been recovered in the Pacific Islands.
These nine novel strains fall into the most ancient lineages in the family tree of M. leprae, branches 5 and 0. The last time that these branches shared a common ancestor was almost 4,000 years ago.
These findings suggest that leprosy was not introduced to the Pacific Islands during colonialism in the 1800s, as previously thought. Colonialism may have brought subsequent strains of leprosy to the Pacific Islands, but those strains would belong to different branches of the evolutionary tree.
“Specifically, our results suggest that leprosy was probably present in the Pacific prior to European contact and likely spread via trade/voyaging networks,” said Anne Stone, ASU Regents Professor and senior co-author of the research.
Leprosy is a chronic infectious disease that can be fatal. While it is still around today, it can be treated with antibiotics.
The tissue samples for this study came from the Hawaiian Pathologists’ Laboratory. Blevins and Crane received the samples at ASU’s Lab of Molecular Anthropology, selected and adapted a method for extracting DNA, analyzed data and wrote the manuscript.
The research team also conducted bioinformatics work, comparing their data with publicly available M. leprae genomic data records. (See Figure 1 from the research paper.)
This additional data also helped Blevins and Crane analyze relationships between strains and create phylogenetic trees. Crane called them the “family trees” of the pathogen, showing the evolutionary history of the various strains.
ASU graduate students lead the study
Blevins and Crane are both doctoral students at ASU — Blevins in anthropology at the School of Human Evolution and Social Change, and Crane in evolutionary biology at the School of Life Sciences. They designed the study and executed the laboratory work and data analysis.
Blevins and Crane come from different areas of primary research and past academic work, but now they both want to know where diseases come from.
Blevins’ work and research during her undergraduate and graduate studies focused on osteology (studying bones) and paleopathology (studying ancient disease). This background provided a good foundation for a transition into genomics research.
“You can only tell so much from bones,” Blevins said. “They’re like a signpost saying ‘something was causing disease here,’ so getting into genomics helps better piece together what was going on.”
Crane comes from a background in molecular biology. She originally wanted to become a veterinarian and study wildlife diseases, but her research journey has led her to study how new interactions with the environment are affecting humans. She hopes to work in government research or surveillance of modern diseases.
Original concept and implications for future research
This research concept came from Keolu Fox, a native Hawaiian and University of California, San Diego assistant professor. He is working to expand the genome database to include more underrepresented populations.
Crane said future research will include more data, including more samples and different types of samples.
“These nine genomes have formed our understanding of leprosy in the Pacific right now,” Crane said. “We have a much better picture of where it is and what’s going on.”
But, she is interested in gathering data from a wider geographic area. Crane is continuing to research leprosy with the Hawaiian Pathologists’ Laboratory as part of her dissertation, for which she is researching bacterial populations within a host.
The paper “Evolutionary history of Mycobacterium leprae in the Pacific Islands” was published in a special themed issue of the Philosophical Transactions B journal, “Insights into health and disease from ancient biomolecules.”
More Science and technology

Lucy's lasting legacy: Donald Johanson reflects on the discovery of a lifetime
Fifty years ago, in the dusty hills of Hadar, Ethiopia, a young paleoanthropologist, Donald Johanson, discovered what would…

ASU and Deca Technologies selected to lead $100M SHIELD USA project to strengthen U.S. semiconductor packaging capabilities
The National Institute of Standards and Technology — part of the U.S. Department of Commerce — announced today that it plans to…
From food crops to cancer clinics: Lessons in extermination resistance
Just as crop-devouring insects evolve to resist pesticides, cancer cells can increase their lethality by developing resistance to…