Theory guides designed concerted electron-proton transfer for energy conversion
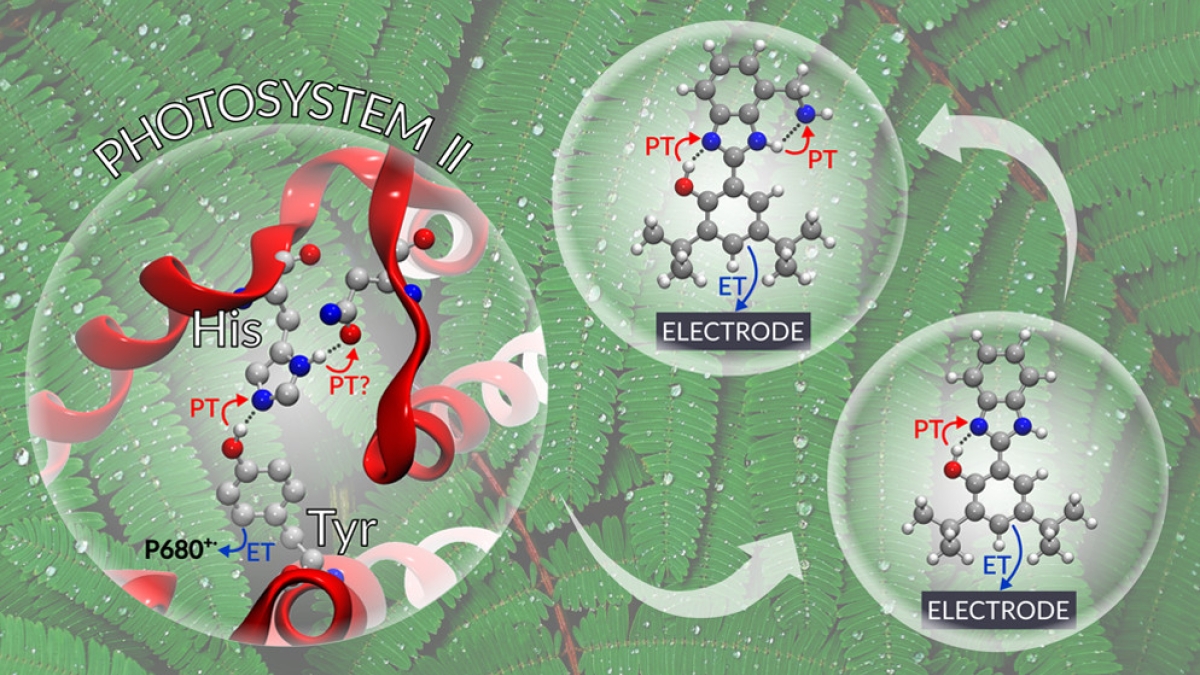
An important contributor to the efficiency in Photosystem II is mediation of charge separation by a tyrosine residue (Tyrz). Oxidation of Tyrz as part of the charge separation process occurs in concert with transfer of a proton from the oxidized tyrosine to a neighboring histidine (His) residue.
This concerted electron-proton transfer not only promotes charge separation, but also generates a proton with the thermodymanic potential to drive water oxidation. Proton-coupled electron transfer is thus critical to the overall light energy conversion process. In turn, understanding how to design and control concerted proton-electron transfer is also important in the development of artificial methods for solar fuel production based on Photosystem II, and other technologies that require managing proton activity.
Photosystem II is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It captures photons and via charge-separation uses the energy to oxidize water. The TyrZ-His redox-relay pair couples proton transfer to the redox process between P680 and the water oxidizing catalyst in photosystem II.
In a recent collaborative study with researchers from the University of Illinois, Université Paris-Sud and the University of Virginia, Tom Moore, Ana Moore and Devens Gust of Arizona State University's School of Molecular Sciences recently showed how theory can be used to guide the design of a concerted electron–proton transfer process, inspired by the a TyrZ-His pair in Photosystem II.
The study used artificial redox relays based on phenol/benzimidazole pairs to test the theory that a concerted two proton transfer process associated with the electrochemical oxidation of the phenol would take place when the benzimidazole substituents are strong proton acceptors, such as primary or tertiary amines. Theory also predicts a decrease in the redox potential of the phenol and a small kinetic isotope effect (KIE). Electrochemical, spectroelectrochemical and KIE experimental data provide strong evidence to support these predictions. The work was described in a recent paper in ACS Central Science.
As Tom Moore explains, “Inspired by nature, and working closely with Professor Hammes-Schiffer's group at the University of Illinois, we designed a proton coupled electron transfer construct and demonstrated that two proton transfer steps could be driven by one electron transfer step. … This work could be important in solar energy conversion to fuels, in the construction of artificial neural networks for artificial intelligence, and in general for the use of electricity to power proton currents and support lifelike processes in artificial cells.”
This study is significant because it shows that theory can be used to guide the design of artificial systems for energy conversion. Protons are important in myriad ways for solar fuel production, so the results of this study also have implications for managing proton activity to optimize efficiency at energy conversion sites involving water oxidation and reduction.
This work was supported by a DOE grant to Gust, Moore and Moore and also involved SMS researchers S. Jimena Mora, Matias Villalba, Marley E. Tejeda-Ferrari, Paul A. Liddell and Brian R. Cherry.
More Science and technology

Indigenous geneticists build unprecedented research community at ASU
When Krystal Tsosie (Diné) was an undergraduate at Arizona State University, there were no Indigenous faculty she could look to…

Pioneering professor of cultural evolution pens essays for leading academic journals
When Robert Boyd wrote his 1985 book “Culture and the Evolutionary Process,” cultural evolution was not considered a true…

Lucy's lasting legacy: Donald Johanson reflects on the discovery of a lifetime
Fifty years ago, in the dusty hills of Hadar, Ethiopia, a young paleoanthropologist, Donald Johanson, discovered what would…